Vaccine Adjuvants Market By Type(Particulate, Pathogen, Adjuvant emulsion, Combination, Others), By Application(Infectious diseases, Cancer, Others), By Administration(Intramuscular, Intradermal, Intranasal, Oral, Others), By Region And Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, And Forecast 2024-2033
-
3318
-
March 2024
-
169
-
-
This report was compiled by Trishita Deb Trishita Deb is an experienced market research and consulting professional with over 7 years of expertise across healthcare, consumer goods, and materials, contributing to over 400 healthcare-related reports. Correspondence Team Lead- Healthcare Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Quick Navigation
Report Overview
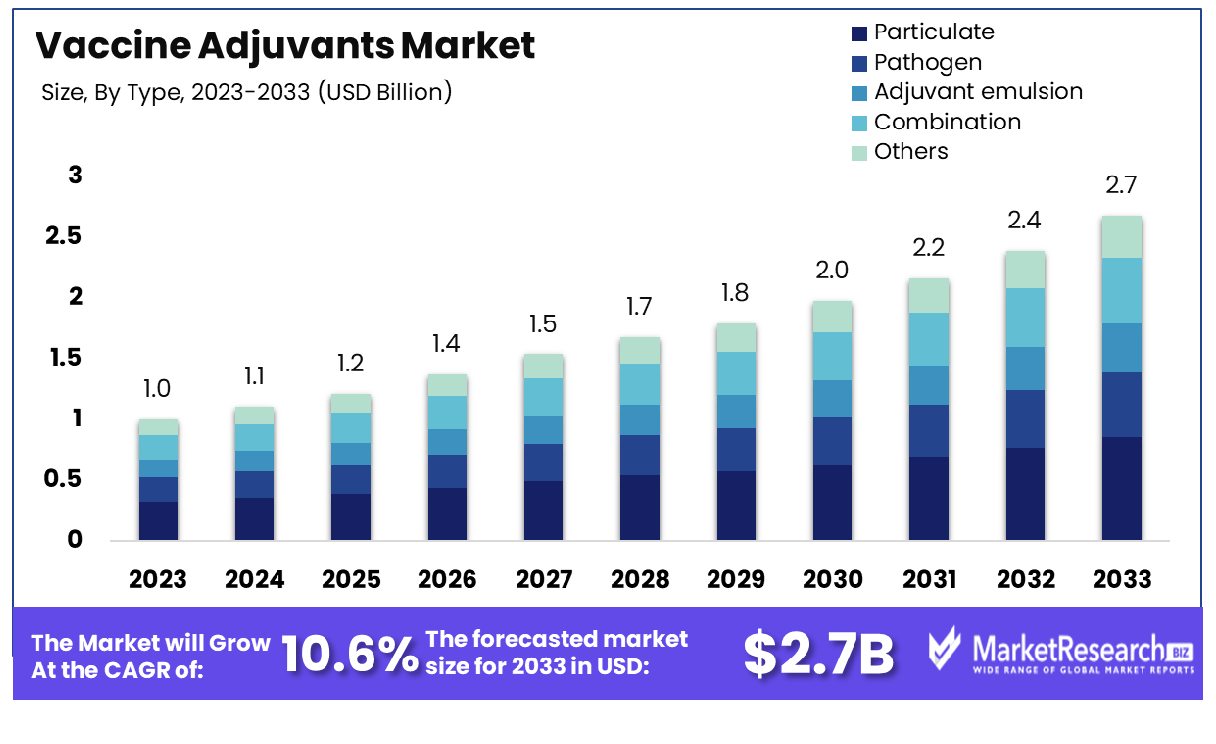
The global Vaccine Adjuvants Market was valued at USD 1.0 billion in 2023, It is expected to reach USD 2.7 billion by 2033, with a CAGR of 10.6% during the forecast period from 2024 to 2033.
The surge in various types of diseases, the rise in numbers of vaccine approvals, and different types of government initiatives are some of the main key driving factors for the vaccine adjuvants market.
Vaccine adjuvants are defined as the substances added to vaccines to improve the body’s immune response to the antigens, which is the main component that activates the immune system to generate antibodies. Adjuvants work by promoting a sturdy and more prolonged immune response that leads to more efficacy and effectiveness of the vaccines. They can also allow for less amount of antigens that can be used in each vaccine dose which can help in vaccine production and supply.
Adjuvants function by stimulating immune cells and signaling pathways, activating the production of cytokines and chemokines, and promoting the maturation of antigen-presenting cells. This outcome in a more enhanced and durable immune response that comprises the production of higher levels of antibodies aluminum salts, oil-in-water emulsion, and toll-like receptor agonists. Adjuvants play an important role in vaccine creation by making vaccines that are safer, more effective, and potentially need fewer doses for optimum protection.
MIT news in March 2024, highlights that MIT engineers have developed a nanoparticle vaccine that is made from a metal-organic framework known as ZIF-8. It is coated with the SARS-CoV-2 receptors that bind protein and adjuvants known as Gdq. Moreover, Evonik in October 2023, highlights that Enovik has launched GMP-quality plant-based squalene known as phytofluene, which is a non-animal-derived squalene. The firm focuses on changing more than 70% of its portfolio to generation solutions by 2032.
The recent developments in vaccines highlight the great importance of adjuvants. These compounds augment the immune responses and make the development of more potent and long-lasting vaccines. As infectious threats increase and rapid vaccine production is required, adjuvants help expedite vaccine development processes. Moreover, they simplify the administration of smaller antigen doses, potentially decreasing manufacturing expenses and improving world vaccine accessibility.
With their potential to improve vaccine effectiveness and enhance immune protection, adjuvants display an important component in identifying the emergence of public health risks. The demand for vaccine adjuvants will increase due to its requirement in vaccine developments that will help in market expansion in the coming years.
Key Takeaways
- Market Growth: Vaccine Adjuvants Market was valued at USD 1.0 billion in 2023, It is expected to reach USD 2.7 billion by 2033, with a CAGR of 10.6% during the forecast period from 2024 to 2033.
- By Type: Particulate adjuvants lead due to enhanced immune response and formulation versatility.
- By Application: Infectious diseases drive demand, necessitating effective adjuvants for vaccine development.
- By Administration: Intramuscular administration prevails, ensuring efficient vaccine delivery and immune activation.
- Regional Dominance: In North America, the vaccine adjuvants market dominates with a commanding 42.5% market share.
- Growth Opportunity: Global vaccine adjuvants market growth in 2023 was driven by expanding vaccination programs, technological advancements enhancing product development, and rising focus on immunotherapy, fueling market expansion.
Driving factors
Escalating Demand for Enhanced Vaccine Efficacy
A key force driving the vaccine adjuvants market is an increasing demand for more efficacious vaccines. Adjuvants play a pivotal role in enhancing the immune response to vaccines, thereby amplifying their effectiveness. With the rising prevalence of infectious diseases and the emergence of novel pathogens, there is a growing need for vaccines that offer superior protection.
This demand is further fueled by the ongoing efforts to combat global health threats such as influenza, COVID-19, and various types of cancers. As a result, vaccine manufacturers are increasingly incorporating adjuvants into their formulations to boost the immune system's response, thereby driving the expansion of the market.
Advancements in Vaccine Formulation Technologies
Another significant driver contributing to the growth of the vaccine adjuvants market is the continuous advancements in vaccine formulation technologies. Researchers and pharmaceutical companies are constantly innovating to develop novel adjuvants that offer improved safety profiles, enhanced efficacy, and broader applicability. This includes the development of adjuvants capable of inducing robust and long-lasting immune responses while minimizing adverse reactions.
Furthermore, adjuvant delivery systems such as nanoparticles and liposomes have broadened the potential of adjuvant-based vaccine formulations even further. These technological innovations not only drive market growth but also pave the way for the development of next-generation vaccines with superior protective capabilities.
Rising Investments in Vaccine Research and Development
Global investments in vaccine research and development (R&D) initiatives serve as an integral factor in driving market expansion for vaccine adjuvants. Governments, non-profit organizations, and private sector entities are allocating significant funds towards creating new vaccines or improving existing ones - further expanding the vaccine adjuvants market.
These investments are driven by the pressing need to address public health challenges, enhance pandemic preparedness, and achieve global immunization goals. As such, there has been an increased focus on discovering novel adjuvants and adjuvant-based vaccine formulations to address the limitations associated with conventional vaccines. This surge in R&D investments not only accelerates innovation but also fosters collaborations between academia, industry, and regulatory bodies, thereby fostering market growth.
Restraining Factors
Stringent Regulatory Requirements
Vaccine Adjuvants Market regulators impose stringent requirements to approve and market vaccine adjuvants, thus serving as a major hindrance. These requirements include rigorous testing for safety, efficacy, and compatibility with existing vaccines.
Moreover, the lengthy and complex approval processes often lead to delays in market entry, hindering the growth potential of vaccine adjuvants. Companies investing in research and development face substantial costs and uncertainties associated with meeting regulatory standards, which can deter innovation and limit market expansion.
Limited Understanding of Adjuvant Mechanisms
One factor hindering growth in the Vaccine adjuvant market is our limited understanding of adjuvant mechanisms. Despite their proven efficacy in enhancing immune responses, the precise mechanisms of action for many adjuvants remain poorly understood. This lack of clarity hampers the development of new and improved adjuvants, as researchers face challenges in optimizing formulations and predicting outcomes.
Additionally, uncertainties surrounding the long-term safety profiles of adjuvants contribute to cautious adoption by healthcare providers and vaccine manufacturers. Without a comprehensive understanding of adjuvant mechanisms, stakeholders may hesitate to invest in the widespread utilization of these critical components in vaccine formulations.
By Type Analysis
Particulate-dominated segment leads the Vaccine Adjuvants Market, owing to their efficacy in enhancing immune responses.
Particulate was the market leader in 2023 for the Type segment of the Vaccine Adjuvants Market. This category encompassed various adjuvant types essential to vaccine efficacy and robust immune responses; Particulate adjuvants were distinguished by their particulate nature; these substances include nanoparticles, microparticles, or liposomes designed to mimic pathogens while efficiently delivering vaccine antigens.
Pathogen-based adjuvants represent another significant subset within this segment. Derived from components of pathogens such as bacterial or viral particles, these adjuvants stimulate innate immune responses, augmenting antigen presentation and antibody production. Their ability to closely mimic infectious agents while maintaining safety makes them valuable assets in vaccine formulations.
Adjuvant emulsions constitute yet another pivotal category. Comprising oil-in-water or water-in-oil emulsions, these adjuvants possess superior antigen solubilization properties, facilitating sustained release and prolonged immune stimulation. Their formulation versatility enables customization to suit diverse vaccine requirements.
Furthermore, the Combination subgroup merges multiple adjuvant types to synergistically enhance vaccine efficacy. This approach aims to maximize immunogenicity and broaden protective immune responses by leveraging the complementary mechanisms of different adjuvants.
Lastly, the Others category encompasses emerging adjuvant technologies not classified under the aforementioned groups. This segment encapsulates innovative formulations, novel delivery systems, and unconventional adjuvant candidates, reflecting ongoing advancements in vaccine adjuvant research and development.
By Type Analysis
Infectious diseases dominate the application segment, driving the demand for vaccine adjuvants to bolster immunogenicity.
In 2023, infectious diseases dominated the "By Application" segment of the Vaccine Adjuvants Market. Due to rising incidence rates and efforts made towards creating effective vaccines, infectious disease vaccination has led to an upsurge in demand for adjuvants used as adjuvants play an integral role in increasing vaccine efficacy by boosting immune responses thereby protecting various infectious agents.
Furthermore, the increasing incidence of infectious diseases globally, along with the emergence of novel pathogens and antimicrobial resistance, has underscored the urgency for effective vaccination strategies. As a result, stakeholders in the healthcare sector are increasingly investing in research and development activities aimed at advancing vaccine formulations and adjuvant technologies.
Moreover, governments and international organizations are actively promoting vaccination campaigns to mitigate the burden of infectious diseases and prevent outbreaks. This collective effort has contributed substantially to the expansion of the vaccine adjuvants market within the infectious disease segment.
In addition to infectious diseases, cancer represents another key application area driving demand for vaccine adjuvants. Immunotherapy as a promising approach to cancer treatment has resulted in an explosion of cancer vaccine research and development. Adjuvants play a crucial role in enhancing the immunogenicity of these vaccines, thereby augmenting the body's immune response against cancer cells.
Looking ahead, the "Others" category within the "By Application" segment of the Vaccine Adjuvants Market encompasses a diverse range of therapeutic areas beyond infectious diseases and cancer. This segment includes applications such as autoimmune diseases, allergies, and chronic conditions, where adjuvants hold the potential for improving vaccine efficacy and disease management.
By Type Analysis
Intramuscular administration emerges as the dominant segment, offering effective delivery and immune system activation for vaccine adjuvants.
Intramuscular led the By Administration segment of the Vaccine Adjuvants Market in 2023. Intramuscular administration has long been revered for its ability to induce robust immune responses, making it a preferred route of vaccine delivery. This segment includes various other routes such as Intradermal, Intranasal, Oral, and others each offering distinct advantages and challenges.
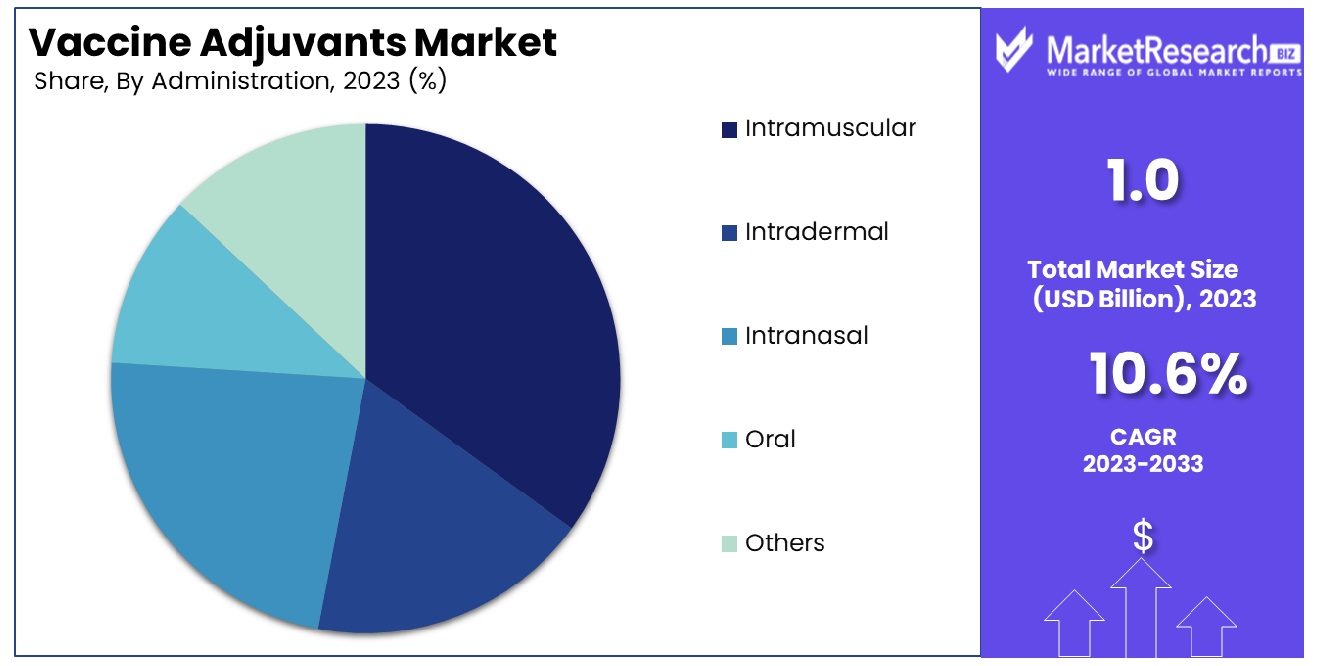
Intramuscular administration, characterized by the injection of vaccines into the muscle tissue, accounted for the largest share of the market. This can be attributed to several factors, including the well-established safety profile, ease of administration, and widespread acceptance among healthcare providers and patients alike. Additionally, the efficacy of many vaccines delivered via the intramuscular route has been extensively studied and documented, further bolstering its market dominance.
The Intradermal route, involving the injection of vaccines into the dermis layer of the skin, also held a notable market share. This method has gained traction in recent years due to its potential for dose-sparing and enhanced immune response, particularly in certain populations such as the elderly. However, challenges such as proper technique and potential skin reactions have tempered its widespread adoption.
Intranasal and Oral administration routes, offering non-invasive alternatives to injections, have garnered increasing interest in the vaccine adjuvants market. These routes hold promise for improved patient compliance and ease of delivery, particularly in pediatric and needle-phobic populations. However, technological advancements and regulatory considerations continue to influence their market penetration.
The "Others" category encompasses emerging administration routes, such as transdermal and mucosal delivery, which are undergoing exploration for their potential in vaccine adjuvant applications. While these approaches present novel opportunities, further research and development are needed to realize their full market potential.
Key Market Segments
By Type
- Particulate
- Pathogen
- Adjuvant emulsion
- Combination
- Others
By Application
- Infectious diseases
- Cancer
- Others
By Administration
- Intramuscular
- Intradermal
- Intranasal
- Oral
- Others
Growth Opportunity
Expanding Vaccination Programs Drive Market Growth
In 2023, the global vaccine adjuvants market witnessed significant growth opportunities propelled by several key factors. Firstly, the expansion of vaccination programs worldwide played a pivotal role. Governments and health organizations across the globe intensified their efforts to combat infectious diseases, leading to an increased demand for vaccines.
With the growing emphasis on preventive healthcare and the need to address emerging infectious threats, the adoption of adjuvanted vaccines surged. Adjuvants, known for enhancing vaccine efficacy and durability of immune responses, became indispensable in the development of effective vaccines against a spectrum of diseases.
Technological Advancements Enhance Product Development
Furthermore, technological advancements in vaccine formulation and adjuvant delivery methods presented promising avenues for market growth. Innovations such as novel adjuvant platforms and advanced delivery systems enabled the development of next-generation vaccines with improved safety profiles and enhanced immunogenicity.
The integration of adjuvant technologies with recombinant antigen design and vaccine delivery systems propelled the development of targeted and tailored vaccine solutions. These advancements not only expanded the scope of vaccine development but also addressed challenges associated with vaccine stability, storage, and administration, thereby driving market expansion.
Rising Focus on Immunotherapy Fuels Market Expansion
Moreover, the escalating focus on immunotherapy and personalized medicine contributed to the burgeoning growth of the vaccine adjuvants market. Immunotherapeutic approaches, including cancer vaccines and therapeutic vaccines for chronic diseases, garnered increasing attention from researchers and pharmaceutical companies.
Adjuvants played a pivotal role in enhancing the immunogenicity of therapeutic vaccines, thereby improving patient outcomes and treatment efficacy. With ongoing research endeavors aimed at harnessing the immune system's potential to combat diseases, the demand for adjuvanted vaccines in the field of immunotherapy is poised to soar, presenting lucrative growth opportunities for market major players.
Latest Trends
Rising Demand for Novel Adjuvant Technologies
The advent of innovative adjuvant technologies marks a pivotal shift in vaccine formulation strategies. Manufacturers are increasingly exploring novel adjuvants, such as lipid nanoparticles, toll-like receptor agonists, and virus-like particles, to enhance vaccine efficacy. These next-generation adjuvants offer improved immunogenicity, antigen-sparing effects, and enhanced cross-protection, addressing unmet needs in vaccine development.
The market embraces this trend as stakeholders prioritize the development of adjuvants capable of augmenting vaccine responses, especially against emerging pathogens and evolving viral variants. Consequently, collaborations between biopharmaceutical companies and research institutions intensify, fostering a fertile ground for breakthrough advancements in vaccine adjuvant technology.
Focus on Personalized Vaccines and Therapeutic Applications
With the advent of precision medicine, personalized vaccines emerge as a promising frontier in healthcare. The integration of adjuvants into personalized vaccine formulations enables tailored immune responses, catering to individual patient profiles and disease susceptibilities. Additionally, the therapeutic potential of vaccine adjuvants extends beyond prophylactic vaccination, encompassing therapeutic vaccines for chronic diseases and cancer immunotherapy.
This paradigm shift towards personalized medicine and therapeutic applications underscores the versatility of vaccine adjuvants, driving market expansion and diversification. As stakeholders harness the power of personalized vaccines and therapeutic modalities, the global vaccine adjuvants market enters a transformative phase, poised for sustained growth and innovation.
Regional Analysis
In North America, the vaccine adjuvants market holds a dominant 42.5% share.
In the global vaccine adjuvants market, North America emerges as the dominant region, commanding a substantial share of approximately 42.5%. The region's dominance is propelled by factors such as robust healthcare infrastructure, significant investments in research and development, and high adoption rates of advanced medical technologies. Moreover, favorable government initiatives aimed at promoting vaccination programs and ensuring public health bolster the market growth in North America.
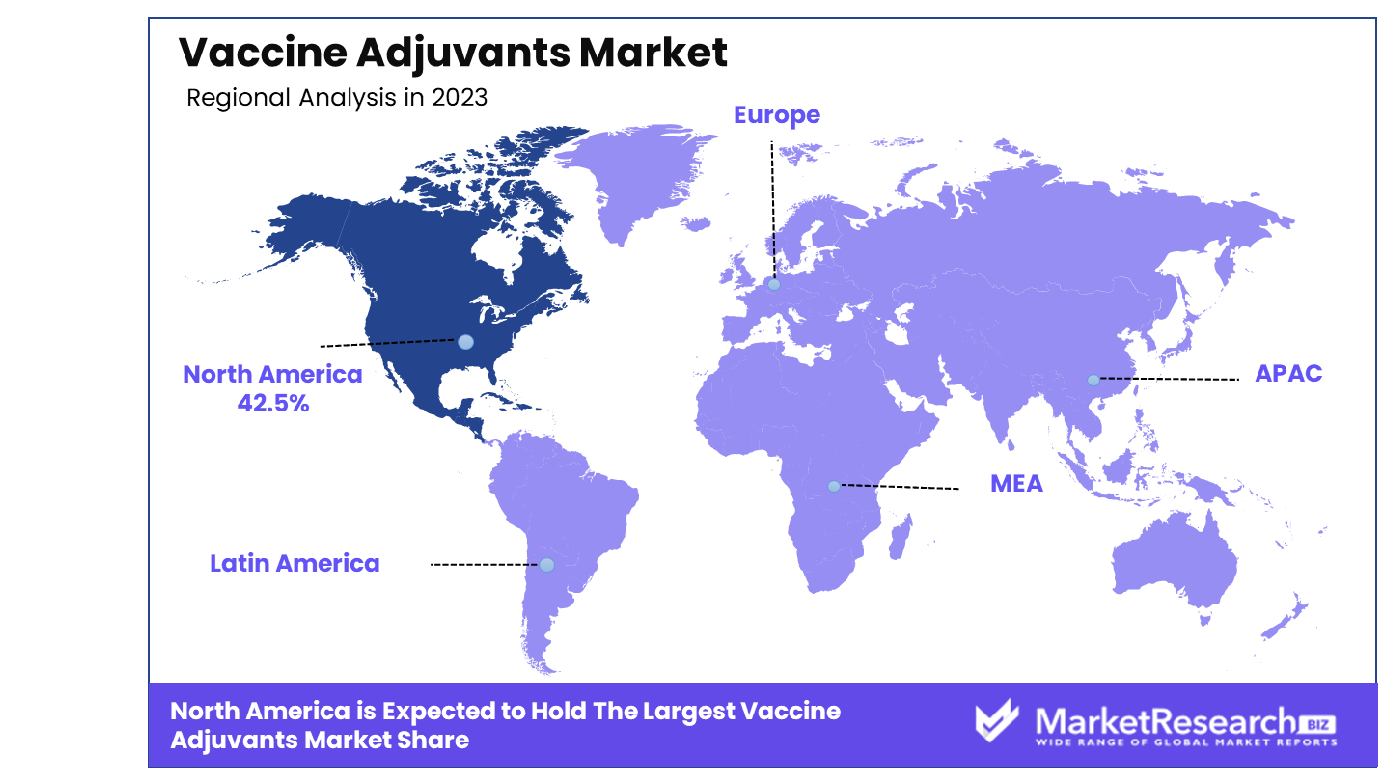
Europe stands as another key region in the vaccine adjuvants market, characterized by a well-established pharmaceutical industry and a strong emphasis on preventive healthcare measures. The region benefits from a large patient pool, increasing prevalence of infectious diseases, and rising awareness regarding the importance of vaccination, thereby driving the demand for adjuvants.
In the Asia Pacific, rapid economic development, expanding healthcare expenditure, and growing initiatives by governments to improve immunization coverage contribute to the market's growth trajectory. Additionally, the region's large population base presents lucrative opportunities for market major players, especially in densely populated countries like China and India.
The Middle East & Africa and Latin America regions are witnessing steady growth in the vaccine adjuvants market, albeit at a relatively slower pace compared to other regions. Factors such as increasing investments in healthcare infrastructure, rising awareness about vaccination programs, and expanding access to immunization services contribute to the market's expansion in these regions.
Key Regions and Countries
North America
- The US
- Canada
- Rest of North America
Europe
- Germany
- France
- The UK
- Spain
- Netherlands
- Russia
- Italy
- Rest of Europe
Asia-Pacific
- China
- Japan
- Singapore
- Thailand
- South Korea
- Vietnam
- India
- New Zealand
- Rest of Asia Pacific
Latin America
- Mexico
- Brazil
- Rest of Latin America
Middle East & Africa
- Saudi Arabia
- South Africa
- UAE
- Rest of Middle East & Africa
Key Players Analysis
In the dynamic landscape of the global Vaccine Adjuvants Market in 2023, several key market players stand out for their pivotal contributions and market influence. Among these, Brenntag Biosector A/S emerges as a leading force, leveraging its extensive expertise in adjuvant technologies to propel advancements in vaccine development. With a robust portfolio of adjuvant solutions tailored to enhance vaccine efficacy and safety, Brenntag Biosector A/S plays a vital role in driving innovation and addressing unmet needs in the market.
CSL Limited commands significant attention as well, renowned for its comprehensive range of adjuvants designed to optimize immune responses and improve vaccine performance. Backed by a rich heritage of scientific excellence and strategic partnerships, CSL Limited continues to shape the landscape of vaccine adjuvants through pioneering research and development initiatives.
Air Liquide S.A. also emerges as a key player, offering cutting-edge adjuvant technologies and specialized services to support vaccine manufacturers worldwide. With a steadfast commitment to sustainability and innovation, Air Liquide S.A. contributes to the expansion of the vaccine adjuvants market while ensuring environmental responsibility and regulatory compliance.
Additionally, companies such as Agenus Inc., Novavax Inc., and InvivoGen Inc. showcase notable prowess in adjuvant research and development, driving advancements in vaccine formulation and delivery systems. Their collaborative efforts with industry partners and academic institutions further catalyze innovation and accelerate the pace of vaccine development.
As the global demand for effective vaccines continues to rise, key market players like Brenntag Biosector A/S, CSL Limited, and Air Liquide S.A. remain at the forefront of innovation, shaping the future of the vaccine adjuvants market with their expertise, resources, and unwavering commitment to public health.
Market Key Players
- Brenntag Biosector A/S
- CSL Limited
- Air Liquide S.A.
- Agenus Inc.
- Novavax Inc.
- InvivoGen Inc.
- Avanti Polar Lipids, Inc.
- MVP Laboratories Inc.
- OZ Biosciences
- Croda International PLC
- SPI Pharma Inc.(Associated British Foods plc)
- Thermo Fisher Scientific Inc.
- Biotech Company
- Merck KGaA
- Dynavax Technologies Corporation
Recent Development
- In March 2024, MIT researchers develop a nanoparticle-based vaccine delivery system utilizing metal-organic frameworks (MOFs) to encapsulate and deliver SARS-CoV-2 spike protein while acting as adjuvants, potentially enhancing vaccine efficacy and accessibility.
- In February 2024, Researchers at John Innes Centre developed a heterologous expression system in Nicotiana benthamiana to produce QS-21, a key vaccine adjuvant, potentially enhancing accessibility and allowing for bioengineering of bespoke saponin-based adjuvants.
- In January 2024, Zoetis' Fostera Gold PCV MH, utilizing MetaStim adjuvant, induces robust humoral and cellular immunity against PCV2, ensuring 23 weeks of extended protection, surpassing other vaccines in the market.
Report Scope
Report Features Description Market Value (2023) USD 1.0 Billion Forecast Revenue (2033) USD 2.7 Billion CAGR (2024-2032) 10.6% Base Year for Estimation 2023 Historic Period 2016-2023 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Type(Particulate, Pathogen, Adjuvant emulsion, Combination, Others), By Application(Infectious diseases, Cancer, Others), By Administration(Intramuscular, Intradermal, Intranasal, Oral, Others) Regional Analysis North America - The US, Canada, Rest of North America, Europe - Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe, Asia-Pacific - China, Japan, South Korea, India, New Zealand, Singapore, Thailand, Vietnam, Rest of Asia Pacific, Latin America - Brazil, Mexico, Rest of Latin America, Middle East & Africa - South Africa, Saudi Arabia, UAE, Rest of Middle East & Africa Competitive Landscape Brenntag Biosector A/S, CSL Limited, Air Liquide S.A., Agenus Inc., Novavax Inc., InvivoGen Inc., Avanti Polar Lipids, Inc., MVP Laboratories Inc., OZ Biosciences, Croda International PLC, SPI Pharma Inc.(Associated British Foods plc), Thermo Fisher Scientific Inc., Biotech Company, Merck KGaA, Dynavax Technologies Corporation Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Brenntag Biosector A/S
- CSL Limited
- Air Liquide S.A.
- Agenus Inc.
- Novavax Inc.
- InvivoGen Inc.
- Avanti Polar Lipids, Inc.
- MVP Laboratories Inc.
- OZ Biosciences
- Croda International PLC
- SPI Pharma Inc.(Associated British Foods plc)
- Thermo Fisher Scientific Inc.
- Biotech Company
- Merck KGaA
- Dynavax Technologies Corporation




