Influenza Vaccine Market By Vaccine Type (Quadrivalent and Trivalent), By Technology (Egg-Based and Cell-Based), By Age Group (Paediatric and Adult), By Route of Administration (Injection and Nasal Spray), and By Region, 2023- 2032
-
41810
-
Feb 2022
-
181
-
-
This report was compiled by Trishita Deb Trishita Deb is an experienced market research and consulting professional with over 7 years of expertise across healthcare, consumer goods, and materials, contributing to over 400 healthcare-related reports. Correspondence Team Lead- Healthcare Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Quick Navigation
Report Overview
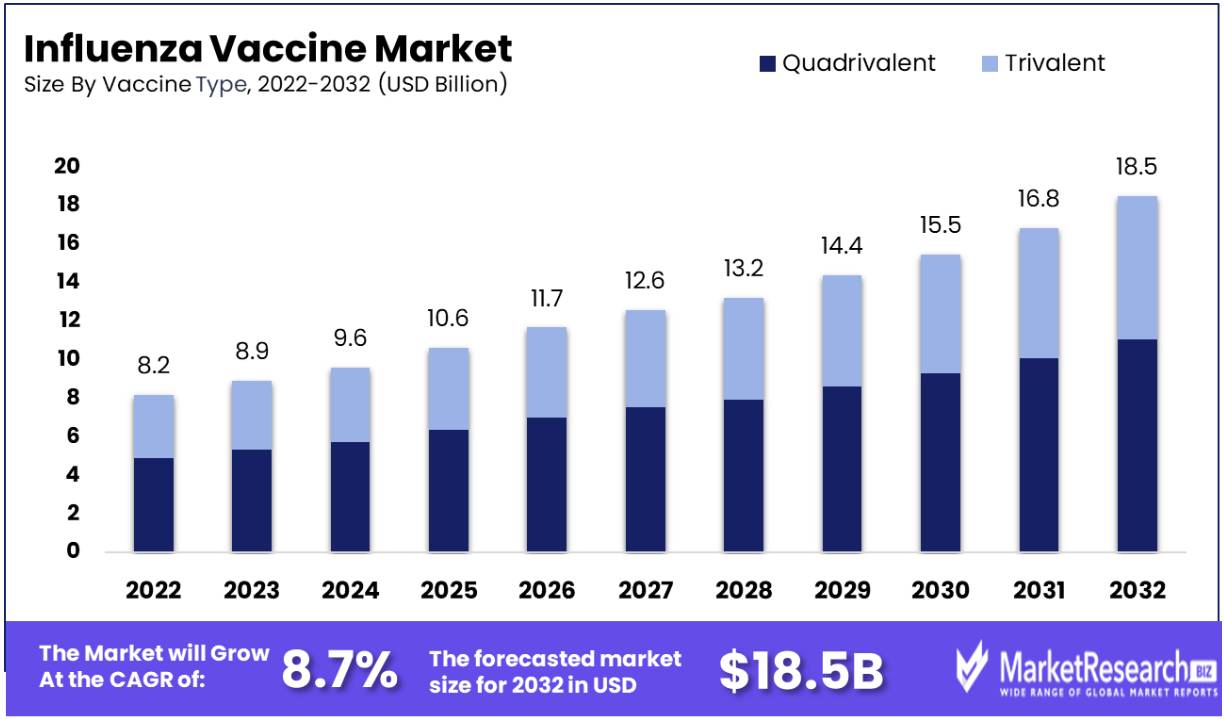
The global influenza vaccine market reached a value of US$ 8.2 Billion in 2022. It expects the market to reach US$ 18.5 Billion by 2032, exhibiting a CAGR of 8.7% during 2023-2032.
Influenza, commonly known as the flu, is a global health concern, with regional outbreaks leading to thousands of deaths annually. To mitigate this risk, influenza vaccines are administered through injection or nasal spray, containing weakened or inactivated virus strains. This prompts the immune system to produce antibodies, reducing infection likelihood and symptom severity.
Major recipients include young children, the elderly, and individuals with primary health conditions. In 2022, the WHO reported 3.0 to 5.0 million severe cases, resulting in 650,000 worldwide deaths. The anticipation of more common outbreaks and epidemics is probable to boost vaccine sales in the years ahead.
The market currently offers several licensed seasonal influenza vaccines, recommended by reputable organizations like the CDC and WHO. Early vaccination is advocated by government agencies to maximize protection during flu season. However, developing vaccines against specific strains remains an ongoing challenge for pharmaceutical companies. The escalating demand for influenza vaccines is projected to drive market growth.
These vaccines typically cover three influenza virus types: A with H3N2, A with H1N1, and B virus strains. The increasing prevalence of influenza epidemics and seasonal outbreaks is projected to bolster product sales in the foreseeable future.
Furthermore, heightened government support and vigilant surveillance at both national and global levels for influenza vaccination programs significantly contribute to market expansion. Substantial investments by leading market players worldwide, coupled with heightened government funding, have facilitated the development and launch of therapeutically effective vaccines, further stimulating growth in the influenza vaccine market.
While the coronavirus pandemic has affected immunization programs, vaccination rates for the flu reached their peak during this period. Various healthcare organizations and governments have actively promoted and provided free flu vaccinations, raising awareness among the population due to concerns about both the flu and COVID-19.
Driving Factors
Increasing Efforts and Support from The Government
Governments worldwide are taking active steps to bolster influenza vaccination efforts by implementing various support measures and initiatives. They are progressively channelling resources into vaccination campaigns to raise awareness about the significance of flu shots and earmarking budgets for the procurement and distribution of vaccines. In many countries, priority groups like children, the elderly, and healthcare professionals have access to subsidized or free vaccination programs, effectively enhancing vaccine availability.
Moreover, government-backed immunization policies enforce mandatory vaccinations in specific settings, such as schools and healthcare facilities, further contributing to the uptake of influenza vaccines. This substantial support from governmental bodies creates an environment conducive to the growth of the influenza vaccine market, ensuring a broader reach across the population. This, in turn, safeguards public health and alleviates the burden of influenza-related illnesses.
Increasing Emphasis On Preventive Healthcare
With a growing awareness of the importance of vaccinations in averting seasonal flu outbreaks, individuals are becoming more proactive in seeking immunization. Additionally, government initiatives and healthcare organizations' concerted efforts in promoting vaccination campaigns have significantly contributed to higher vaccine uptake. Furthermore, advancements in vaccine development technologies have led to the production of more effective and accessible vaccines, bolstering market growth.
Rising Prevalence of Influenza Cases Worldwide
The surge in reported cases, particularly among vulnerable populations such as the elderly and those with chronic illnesses, has prompted an escalated demand for influenza vaccines. Additionally, the threat of potential pandemics and the need for preparedness have heightened the urgency for robust vaccination programs, further propelling market expansion.
Strong Presence of Market Players and Pharmaceutical Companies
They are actively engaged in research and development activities plays a pivotal role in driving the market. Continuous efforts to improve vaccine formulations, enhance efficiency, and expand production capabilities are significant contributors to the growth of the influenza vaccine market.
Restraining Factors
Constant Need for Strain Prediction and Selection
The consistent requirement for strain prediction and selection stands as a critical aspect in the influenza vaccine market. This stems from the nature of the influenza virus, which consistently undergoes mutations. Consequently, there's a need to annually adjust vaccine formulations to effectively combat these evolving strains. However, this process entails a considerable investment of time and resources, presenting a notable challenge and limitation to the market's potential for growth.
Concerns Related to Vaccine Safety and Adverse Effects
The concerns related to vaccine safety and adverse effects may impede uptake rates. Although the benefits of vaccination far outweigh potential risks, public perception and misinformation can lead to vaccine hesitancy, limiting overall market expansion. Addressing these concerns through transparent communication and education is paramount, ensuring that the public is equipped with accurate information regarding the safety and efficacy of influenza vaccines. This concerted effort is instrumental in building trust and fostering confidence in vaccination programs.
Pricing and Accessibility
Despite efforts to increase accessibility, there are regions and demographics where vaccines may not be readily available or affordable. Economic disparities and logistical challenges in distribution can hinder widespread vaccine coverage, particularly in developing countries.
Growth Opportunities
Advancements in Vaccine Formulations and Technologies
One significant growth opportunity in the Influenza Vaccine Market lies in the continual advancements in vaccine formulations and technologies. Research and development efforts aimed at enhancing vaccine effectiveness, safety profiles, and delivery methods can revolutionize influenza prevention. Novel approaches, such as the development of universal vaccines that offer broader protection against multiple strains, hold the potential to significantly impact public health. This opportunity is crucial as it addresses the challenge of strain variability and ensures that vaccines remain effective against evolving influenza viruses.
Expanding Market Reach in Developing Regions
Another vital growth opportunity involves expanding the market's reach in developing regions. There is a substantial untapped potential for influenza vaccination programs in areas where accessibility and awareness are limited. By investing in infrastructure, education, and outreach initiatives, pharmaceutical companies and healthcare organizations can extend the benefits of influenza vaccination to a wider population. This expansion is important as it not only contributes to public health on a global scale but also creates new markets for influenza vaccines, fostering economic growth.
Preparedness for Potential Pandemics
Being prepared for potential pandemics represents a critical growth opportunity in the Influenza Vaccine Market. The COVID-19 pandemic underscored the importance of robust pandemic preparedness plans, which include rapid vaccine development, production, and distribution. Proactive investments in research, manufacturing capacity, and global cooperation can ensure a swift response to emerging influenza threats. This opportunity is of paramount importance as it safeguards public health during unforeseen health crises and underscores the pivotal role of influenza vaccines in global health security.
Latest Trends
Focus on Universal Influenza Vaccines
One prominent trend in the Influenza Vaccine Market is the increasing emphasis on universal influenza vaccines. Unlike traditional vaccines that target specific strains, universal vaccines aim to provide broader protection against a wide range of influenza viruses, including those with the potential for pandemic outbreaks. This trend is significant as it addresses the challenge of strain variability and reduces the need for annual vaccine updates. Universal vaccines have the potential to revolutionize influenza prevention by offering longer-lasting and more comprehensive immunity.
Integration of Adjuvants for Enhanced Effectiveness
Another significant trend involves incorporating adjuvants into influenza vaccine formulations. Adjuvants are compounds added to vaccines to improve the body's immune response. When vaccines contain adjuvants, they can generate a resilient and more enduring immune reaction, mostly in demographics like the elderly, who may display a less vigorous response to standard vaccinations. This trend holds paramount importance as it carries the potential to heighten vaccine effectiveness and bolster protection, particularly among vulnerable population.
Advancements in Vaccine Manufacturing and Production Techniques
A key trend in the Influenza Vaccine Market is the continuous advancement of vaccine manufacturing and production techniques. Innovations such as cell-based and recombinant technologies are becoming more prevalent, offering advantages in scalability, speed, and flexibility in vaccine production. These advancements are essential as they contribute to a more robust and responsive supply chain, enabling a quicker response to outbreaks or emerging strains. Additionally, they enhance the overall efficiency and capacity of vaccine production, ensuring a reliable and timely supply of influenza vaccines.
By Vaccine Type
The quadrivalent vaccines dominates the segment. It offers heightened protection against a wider range of influenza strains. In distinction to traditional trivalent vaccines, which aims three flu strains (two influenzas A strains and one influenza B strain), quadrivalent vaccines includes an additional influenza B strain.
This prolonged coverage holds substantial importance, particularly considering that influenza B viruses often undergo antigenic implication, giving rise to different lineages that may not be adequately covered by trivalent vaccines. By providing an eclectic shield, quadrivalent vaccines prove more effective in thwarting influenza infections and relieving the disease's impact.
Healthcare providers and individuals are displaying a preference for quadrivalent vaccines due to their inclusive coverage, which is driving up demand and consequently, segment growth. The adoption of quadrivalent vaccines has become a custom in various vaccination programs. This outpouring in popularity is prompting manufacturers to devote in their production and distribution, safeguarding they can meet the cumulative global demand for influenza vaccination that is both more effective and encircling.
By Technology Type
Egg-Based Production holds the largest market share. Its approach remains one of the most trusted and frequently employed methods of vaccine production, even amidst a sea of other technological options. It stands as a method where strains of influenza virus are unambiguously refined, separating them from within chicken eggs. This is then followed by a rigorous process of harvesting and further processing to bring about the creation of vaccines.
Despite an assortment of diverse production techniques making strides at this day and age, the egg-based method has continuously proven its worth through times—firmly establishing itself as a safe, scalable, and cost-effective solution to produce vaccines. A considerable segment of major vaccine-producing entities owns comprehensive capacities dedicated to egg-based production. Taking advantage of this infrastructure enables large-scale vaccine creation during times both regular and drastic—like the seasonal flu or unforeseen pandemics.
As demand for innovative and fast-track production of vaccines escalates, there is an increasing tendency to explore newer paradigms of technologies like the cell-based and recombinant modes slightly detached from the conventional. Regardless, the long-established reputation of the egg-based method ensures that it continues to drive segment growth noticeably, especially in demography where it comprises an indispensable production mechanism. Consequently, it also ensures a dependable and consistent worldwide supply of influenza vaccines.
By Age Group Type
Undoubtedly, Paediatric segment rules over the largest market segment. The role of the paediatric sector in driving the influenza vaccine market forward is crucial. Its primary function lies in protecting infants and young children from influenza, which ultimately reduces the virus's spread within communities. The vulnerability of children, especially those under five years old, to severe complications caused by the flu emphasizes the critical importance of vaccination in safeguarding their health and well-being. Paediatric vaccines are specially designed to elicit a strong immune response in young recipients, ensuring robust protection against the disease.
Moreover, vaccinating kids contributes extensively to the status quo of herd immunity, efficaciously curtailing the transmission of the virus to susceptible populations, which include the aged and individuals with compromised immune systems. This underscores the acknowledgment by healthcare carriers, governments, and parents of the pivotal function played through paediatric influenza vaccination.
This heightened recognition translates into an accelerated call for paediatric flu vaccines, therefore driving increase in this phase. Manufacturers are continuously at work, innovating and producing paediatric vaccines that are not most effective safe but also highly effective. This ongoing effort drastically contributes to improved public health results and a discount in the usual burden of influenza.
By Route of Administration
The provision of each injection and nasal spray alternatives plays a pivotal role in accommodating gender preference possibilities and requirements. While the injection remains the commonly applied and favoured method for vaccine delivery, the nasal spray gives a needle-free alternative mainly appealing to kids and people with a worry of needles. This twin approach notably complements typical vaccine reputation and uptake in the end contributing to higher vaccination charges throughout numerous age demographics.
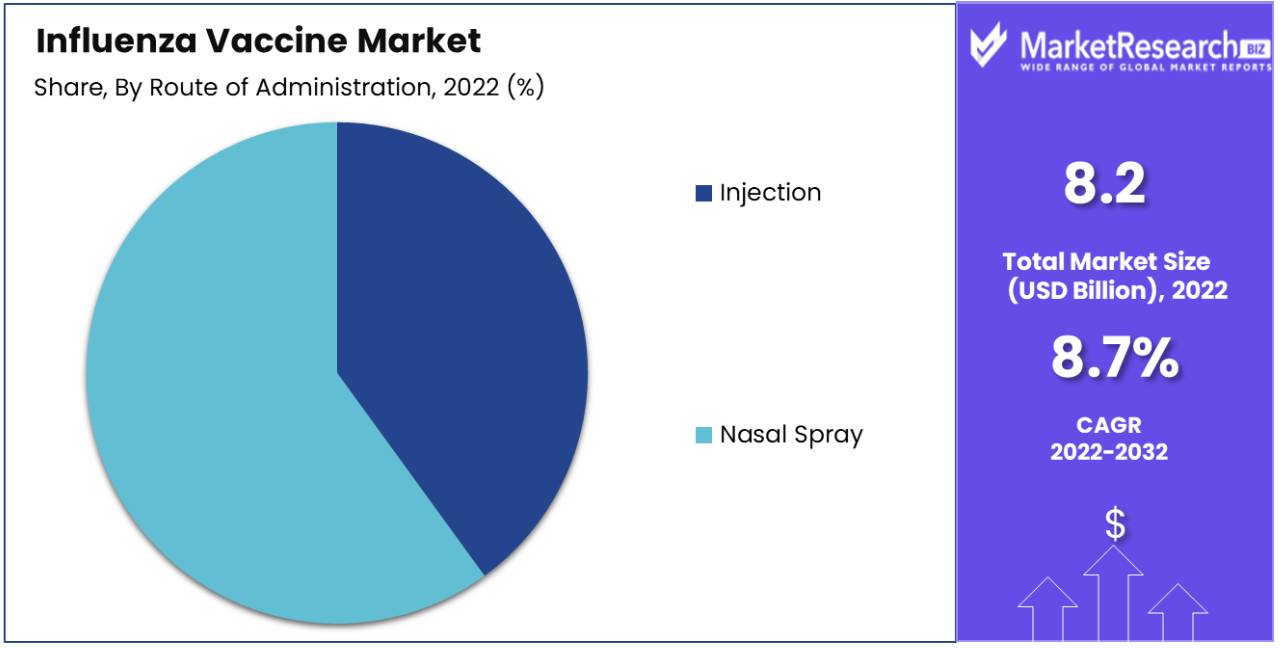
Furthermore, the nasal spray affords a more convenient and green manner of vaccine transport, empowering healthcare vendors to manage vaccines greater expeditiously, mainly in the context of big-scale vaccination campaigns or community-based totally settings. Consequently, the availability of each injection and nasal spray alternatives considerably broadens accessibility and diversifies vaccination alternatives, making sure extra significant coverage for influenza vaccines. Consequently, manufacturers are making huge investments in each transport methods, spotting their critical role in advancing public health and mitigating the effect of influenza.
Key Market Segment
By Vaccine
- Quadrivalent
- Trivalent
By Technology
- Egg-based
- Cell-based
By Age Group
- Paediatric
- Adult
By Route of Administration
- Injection
- Nasal Spray
Regional Analysis
North America has taken the lead in market share, a testament to the region's robust healthcare infrastructure, substantial healthcare expenditure, and advanced research capabilities. These factors collectively create an environment conducive to the development and distribution of vaccines. Furthermore, North America faces a seasonal burden of influenza, compelling healthcare authorities to prioritize vaccination initiatives and campaigns. The proactive approach of the government, including awareness campaigns and subsidized vaccine distribution, significantly contributes to heightened vaccine uptake across the region.
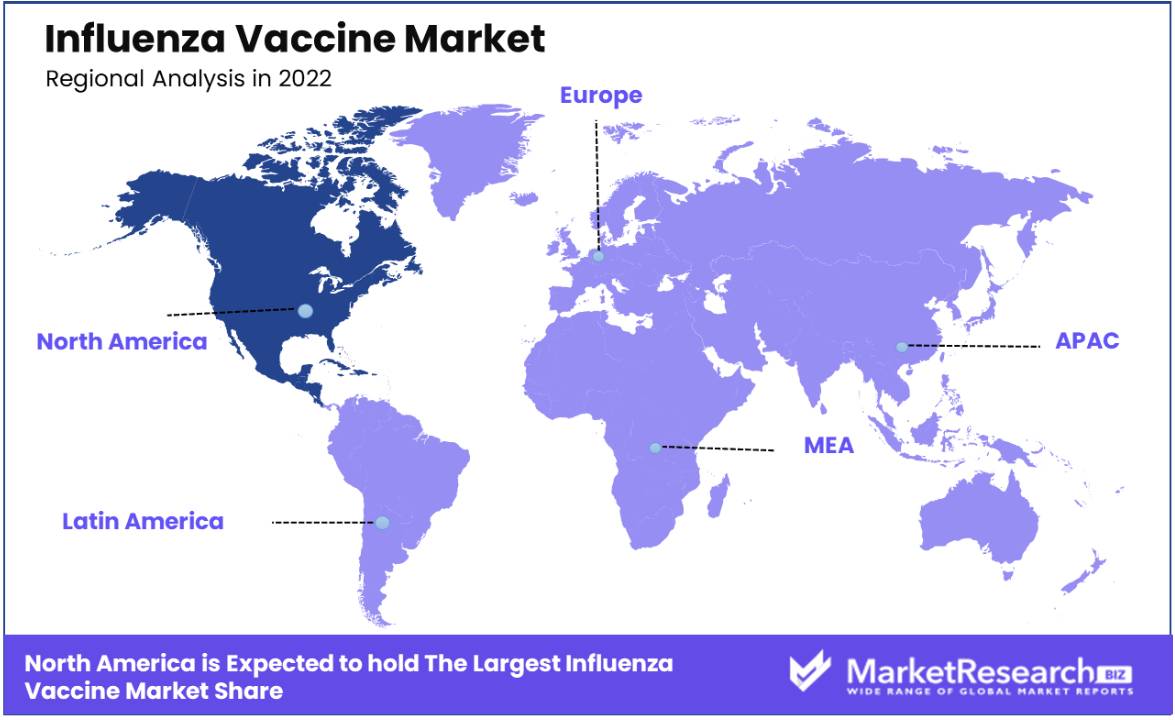
Another pivotal factor is the growing emphasis on preventive healthcare and the heightened awareness among the population regarding the advantages of influenza vaccination. Manufacturers are increasingly recognizing the potential of the North American market and are thus channelling investments into research and production facilities, aligning with the region's specific needs. This strategic approach underscores the commitment to safeguarding public health in North America through effective influenza vaccination initiatives.
Key Regions and Countries
North America
- US
- Canada
Europe
- Germany
- France
- UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Player Analysis
The market is currently experiencing a consistent boom trajectory, driven with the aid of manufacturer’s proactive adoption of innovative strategies geared toward improving vaccine effectiveness, accessibility, and convenience. Notably, the development of superior broadly protective vaccines is a pivotal factor propelling market expansion. Key industry players are intensifying their studies endeavors to create vaccines that provide advanced protection towards more than one influenza lines, thereby reducing the need for common updates and improving common efficacy.
Furthermore, extremely good progress in vaccine production techniques, inclusive of cell-based totally and recombinant technology has facilitated quicker and greater scalable production. This ensures a reliable deliver at some point of seasonal spikes in demand and capacity pandemics. Additionally, concerted efforts are being made to refine vaccine shipping strategies with the introduction of needle-loose options like intradermal or nasal spray vaccines. These innovations play a sizable role in addressing vaccine hesitancy and broadening vaccination insurance ultimately fortifying the worldwide conflict towards influenza and its effect on public fitness.
Anticipated traits in the market encompass the access of latest industry players, heightened collaborations amongst key stakeholders and ongoing product innovations together fostering a weather of healthful competition inside the influenza vaccine enterprise. This dynamic landscape is predicted to similarly increase the sphere and cause stronger answers for influenza prevention.
Key Players in Influenza Vaccine Market
- Abbott Laboratories
- AstraZeneca plc
- CSL Limited
- Daiichi Sankyo Company Limited
- Emergent BioSolutions Inc.
- Hoffmann-La Roche AG
- Gamma Vaccines Pty Ltd
- GlaxoSmithKline plc
- Merck & Co. Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi
- SINOVAC
Recent Developments
- In October 2023, AstraZeneca's Supplemental Biologics License Application (sBLA) for the approval of a self- or caregiver-administered option for FluMist Quadrivalent became granted attractiveness. FluMist, a needle-loose nasal spray, is especially formulated to provide safety against influenza.
- In October 2023, Mylab and Serum Institute of India introduced Nasovac S4, heralding India's inaugural needle-free nasal influenza vaccine. This innovative vaccine incorporates four distinct influenza virus strains and is tailored for individuals aged 2 and above. It endeavors to deliver heightened defense against influenza while augmenting vaccination rates through a more convenient and easily accessible alternative. Currently, the vaccine is available in the private markets of India.
- The Influenza [strain A/H2N3] vaccine, developed by Seqirus, a subsidiary of CSL Ltd, a pharmaceutical company renowned for its expertise in manufacturing and marketing influenza vaccines, antivenoms, and a range of other pharmaceutical products, is currently undergoing Phase I of clinical development. This initial stage is focused on evaluating the vaccine's safety and establishing appropriate dosage levels. The likelihood of approval for this vaccine is anticipated in October 2023.
- On March 2023, the FDA's Vaccines and Related Biological Products Advisory Committee (VRBPAC) made the vital selection concerning the choice of particular influenza virus strains for the vaccine formulation.
Report Scope
Report Features Description Market Value (2022) US$ 8.2 Bn Forecast Revenue (2032) US$ 18.5 Bn CAGR (2023-2032) 8.7% Base Year for Estimation 2022 Historic Period 2016-2022 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Vaccine Type (Quadrivalent and Trivalent), By Technology (Egg-Based and Cell-Based), By Age Group (Paediatric and Adult), By Route of Administration (Injection and Nasal Spray), Regional Analysis North America – The US, Canada, Mexico, Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America, Eastern Europe – Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe, Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe, APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC, Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA Competitive Landscape Abbott Laboratories, AstraZeneca plc, CSL Limited, Daiichi Sankyo Company Limited, Emergent BioSolutions Inc., F. Hoffmann-La Roche AG, Gamma Vaccines Pty Ltd, GlaxoSmithKline plc, Merck & Co. Inc., Novartis AG, Pfizer Inc., Sanofi, SINOVAC, Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Abbott Laboratories
- AstraZeneca plc
- CSL Limited
- Daiichi Sankyo Company Limited
- Emergent BioSolutions Inc.
- F. Hoffmann-La Roche AG
- Gamma Vaccines Pty Ltd
- GlaxoSmithKline plc
- Merck & Co. Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi
- SINOVAC




