
Pharma 4.0 Market By Technology (IoT, AI, Big Data, Blockchain, Cloud Computing, Others), By Application (Drug Manufacturing, Quality Control, Supply Chain Management, Regulatory Compliance), By End-User (Pharmaceutical Companies, Contract Manufacturing Organizations (CMOs), Research Institutions), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
-
50538
-
Aug 2024
-
302
-
-
This report was compiled by Trishita Deb Trishita Deb is an experienced market research and consulting professional with over 7 years of expertise across healthcare, consumer goods, and materials, contributing to over 400 healthcare-related reports. Correspondence Team Lead- Healthcare Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Quick Navigation
Report Overview
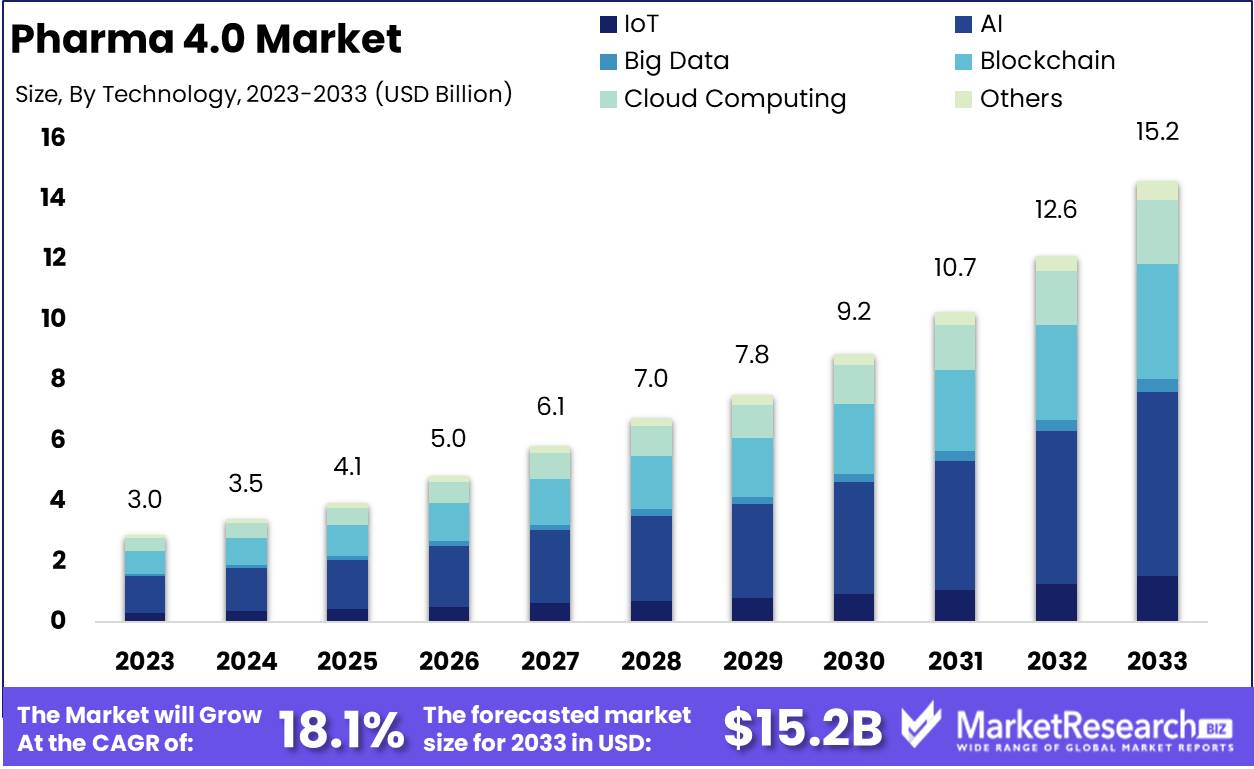
The Global Pharma 4.0 Market was valued at USD 3.0 Bn in 2023. It is expected to reach USD 15.2 Bn by 2033, with a CAGR of 18.1% during the forecast period from 2024 to 2033.
The Pharma 4.0 Market refers to the adoption of advanced digital technologies, automation, and data analytics in the pharmaceutical industry to enhance manufacturing efficiency, product quality, and regulatory compliance. This market includes the integration of smart manufacturing systems, robotics, IoT, and artificial intelligence to streamline production processes and improve operational transparency. Pharma 4.0 represents the industry's shift towards a fully connected and automated manufacturing environment, aimed at meeting the growing demand for precision medicine, reducing time-to-market, and ensuring the highest standards of safety and efficacy.
The Pharma 4.0 Market is rapidly evolving as the pharmaceutical industry embraces advanced digital technologies to enhance manufacturing processes and overall efficiency. In 2022, GlaxoSmithKline (GSK) made a significant investment in a fully automated, sustainable smart manufacturing facility in the UK, highlighting the industry's shift towards Pharma 4.0. This investment underscores the growing importance of automation, data analytics, and digitalization in optimizing production and ensuring regulatory compliance.
One of the key trends within the Pharma 4.0 market is the adoption of robotic aseptic filling systems, which reduce human intervention and minimize errors during the manufacturing process. This technology not only enhances the sterility and safety of pharmaceutical products but also improves manufacturing efficiency by up to 30%. Such advancements are crucial for the production of complex biologics and personalized medicines, where precision and reliability are paramount.
The market is also driven by the increasing demand for real-time data analytics and IoT-enabled smart medical devices that provide greater visibility and control over the manufacturing process. These technologies enable pharmaceutical companies to monitor and optimize production in real-time, leading to reduced waste, lower costs, and faster time-to-market for new drugs.
As the industry continues to shift towards more personalized and precision medicine, the need for flexible and adaptive manufacturing systems will drive further adoption of Pharma 4.0 solutions. Companies that invest in digital transformation and smart manufacturing technologies are well-positioned to lead in this new era of pharmaceutical production, delivering higher quality products more efficiently and at a lower cost.
The Pharma 4.0 market is characterized by significant growth opportunities driven by the integration of advanced digital technologies into pharmaceutical manufacturing. The adoption of automation, robotics, and real-time data analytics is transforming the industry, enabling more efficient, safe, and scalable production processes. Companies at the forefront of this digital revolution will be well-equipped to meet the challenges of the modern pharmaceutical landscape and capitalize on the growing demand for innovative and personalized healthcare solutions.
Key Takeaways
- Market Value: The Global Pharma 4.0 Market was valued at USD 3.0 Bn in 2023. It is expected to reach USD 15.2 Bn by 2033, with a CAGR of 18.1% during the forecast period from 2024 to 2033.
- By Technology: AI constitutes 40% of the market, essential for advancing pharmaceutical manufacturing and process optimization.
- By Application: Drug Manufacturing represents 35%, highlighting the role of AI in improving production efficiency and quality control.
- By End-User: Pharmaceutical Companies make up 50% of the market, leveraging AI and digital technologies for innovation and competitiveness.
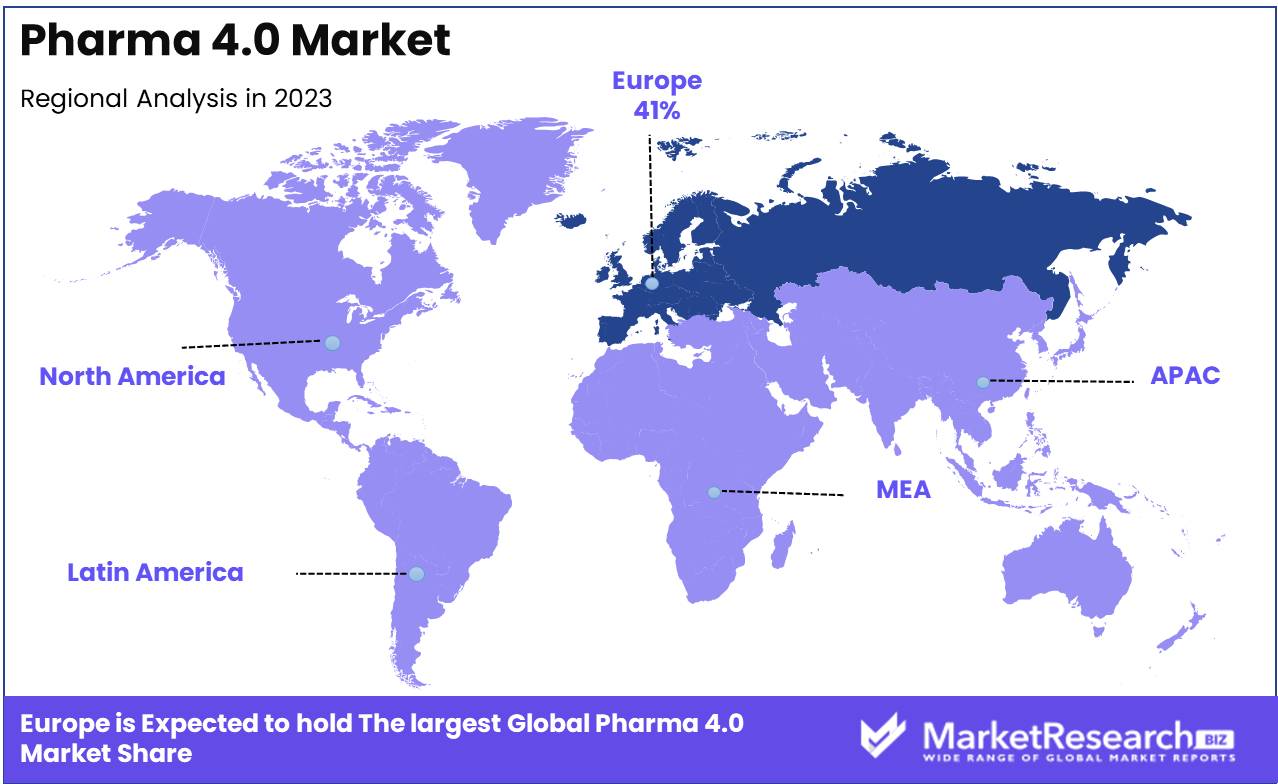
- Regional Dominance: Europe holds a 41% market share, driven by strong regulatory support and investment in pharmaceutical innovation.
- Growth Opportunity: Expanding AI-driven quality assurance and predictive maintenance solutions can significantly enhance operational efficiency and drive market growth in the pharmaceutical industry.
Driving factors
Increasing Adoption of Digital Transformation in the Pharmaceutical Industry Driving Market Growth
The increasing adoption of digital transformation across the pharmaceutical industry is a primary driver for the growth of the Pharma 4.0 Market. As pharmaceutical companies face mounting pressures to improve efficiency, reduce costs, and accelerate time-to-market, the transition to digital processes becomes essential. Digital transformation encompasses the integration of digital technologies into all aspects of pharmaceutical operations, from research and development to manufacturing and distribution.
This shift enables more efficient data management, streamlined production processes, and enhanced regulatory compliance. The widespread embrace of digital transformation is setting the foundation for the Pharma 4.0 Market, creating a fertile environment for the adoption of advanced technologies that further drive innovation and operational excellence in the industry.
Growth in the Use of Advanced Technologies Like AI, IoT, and Blockchain Accelerating Market Expansion
The growth in the use of advanced technologies such as artificial intelligence (AI), the Internet of Things (IoT), and blockchain is significantly accelerating the expansion of the Pharma 4.0 Market. AI is being leveraged for drug discovery, predictive analytics, and optimizing clinical trials, drastically reducing the time and cost associated with bringing new drugs to market. IoT is enhancing real-time monitoring and data collection across pharmaceutical manufacturing, ensuring quality control and compliance with regulatory standards.
Blockchain technology is improving the traceability and security of pharmaceutical supply chains, addressing issues such as counterfeiting and ensuring the integrity of drug products. The convergence of these technologies within the Pharma 4.0 framework is driving unprecedented levels of efficiency, transparency, and innovation, positioning the market for robust growth.
Rising Demand for Personalized Medicine and Precision Manufacturing Boosting Market Adoption
The rising demand for personalized medicine and precision manufacturing is another critical factor contributing to the growth of the Pharma 4.0 Market. Personalized medicine, which tailors treatments to individual patient profiles, requires highly flexible and precise manufacturing processes. Pharma 4.0 enables this through the use of smart manufacturing technologies that allow for the production of small batches of drugs tailored to specific patient needs.
This trend is driving pharmaceutical companies to adopt Pharma 4.0 solutions that can support the production of personalized therapies at scale while maintaining high standards of quality and compliance. As the healthcare industry increasingly moves towards personalized medicine, the demand for Pharma 4.0 technologies is expected to surge, further propelling market growth.
Restraining Factors
High Costs and Complexity of Implementing Pharma 4.0 Solutions Limiting Market Adoption
One of the primary restraining factors for the Pharma 4.0 Market is the high cost and complexity associated with implementing Pharma 4.0 solutions. The integration of advanced technologies such as AI, IoT, and blockchain into pharmaceutical operations requires significant financial investment in infrastructure, software, and training. Small and medium-sized pharmaceutical companies, in particular, may find these costs prohibitive, leading to slower adoption rates compared to larger firms with more substantial resources.
The complexity of integrating these technologies into existing processes can pose significant challenges, requiring specialized expertise and extended implementation timelines. This complexity can lead to disruptions in operations and delays in realizing the potential benefits of Pharma 4.0, thereby limiting its widespread adoption and market growth.
Regulatory and Compliance Challenges Hindering Market Growth
Regulatory and compliance challenges present another significant restraint on the growth of the Pharma 4.0 Market. The pharmaceutical industry is highly regulated, with stringent requirements for quality control, data integrity, and patient safety. The adoption of new technologies within the Pharma 4.0 framework often necessitates updates to compliance protocols and validation processes, which can be time-consuming and costly.
Regulators may be slow to approve or provide guidance on the use of emerging technologies like AI and blockchain, leading to uncertainty and hesitancy among pharmaceutical companies to fully commit to Pharma 4.0 initiatives. This regulatory landscape can create barriers to the adoption of innovative solutions, slowing down the pace of market expansion.
By Technology Analysis
AI technology leads with a 40% share in the Pharma 4.0 Market.
In 2023, AI held a dominant market position in the By Technology segment of the Pharma 4.0 Market, capturing more than a 40% share. Artificial Intelligence (AI) has become a cornerstone in the transformation of the pharmaceutical industry, driving innovation in drug discovery, personalized medicine, and operational efficiency. AI's ability to process vast amounts of data rapidly and accurately has enabled pharmaceutical companies to accelerate drug development timelines, optimize clinical trials, and enhance decision-making processes. The integration of AI into pharma operations has led to significant improvements in productivity and cost-effectiveness, making it the most adopted technology within the Pharma 4.0 framework.
Other technologies such as the Internet of Things (IoT), Big Data, and Blockchain are also crucial to the Pharma 4.0 ecosystem, although they capture smaller market shares compared to AI. IoT facilitates real-time monitoring of manufacturing processes, ensuring quality and compliance, while Big Data analytics helps in managing and interpreting the enormous datasets generated in pharma operations. Blockchain technology is gaining traction for its potential to enhance security and transparency in the supply chain. Cloud computing plays a pivotal role in providing scalable and flexible IT infrastructure, supporting the seamless integration of these technologies across the pharmaceutical value chain.
By Application Analysis
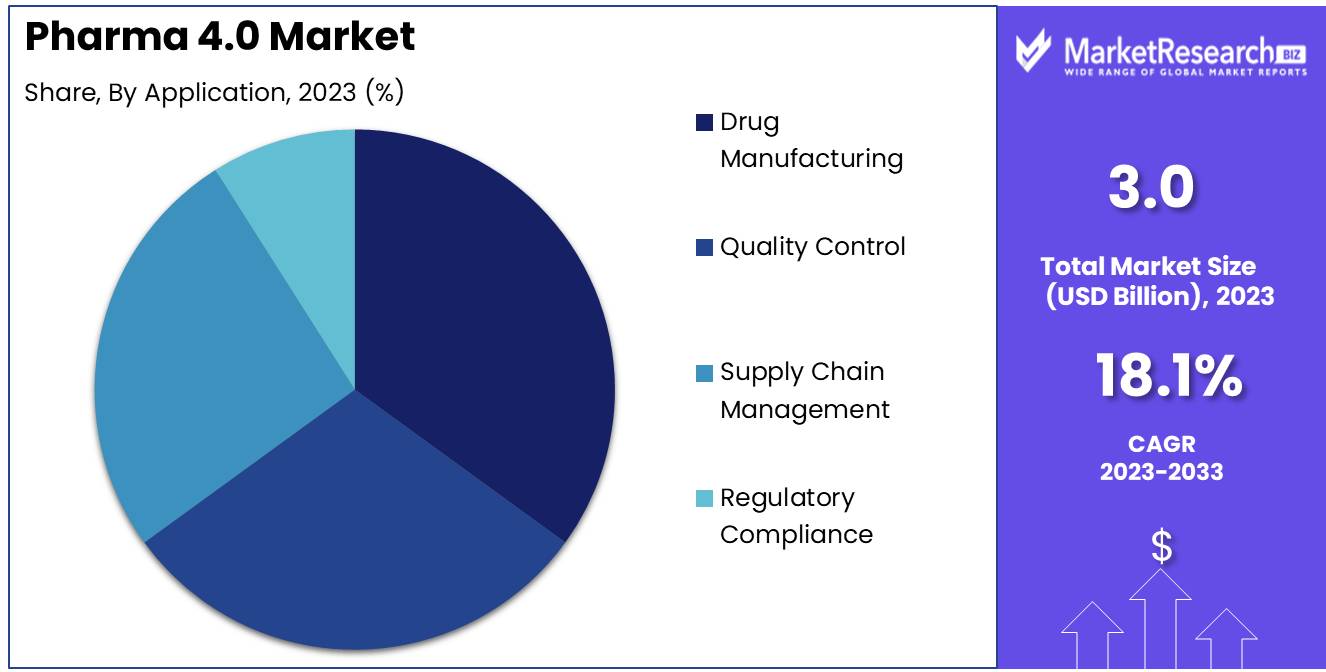
Drug manufacturing applications account for 35% of the Pharma 4.0 Market.
In 2023, Drug Manufacturing held a dominant market position in the By Application segment of the Pharma 4.0 Market, capturing more than a 35% share. The application of Pharma 4.0 technologies in drug manufacturing has revolutionized production processes, leading to enhanced efficiency, consistency, and quality. Advanced technologies like AI and IoT have enabled the automation and optimization of manufacturing workflows, reducing human error and variability in drug production. These innovations have also facilitated the development of continuous manufacturing processes, which are more efficient and cost-effective compared to traditional batch production methods. As pharmaceutical companies strive to meet stringent regulatory standards and demand for high-quality drugs, the adoption of Pharma 4.0 in drug manufacturing is expected to grow further.
Quality control, supply chain management, and regulatory compliance are other key applications of Pharma 4.0 technologies, although they hold smaller shares compared to drug manufacturing. Quality control processes have been enhanced through the use of AI and Big Data analytics, which allow for real-time monitoring and predictive maintenance of equipment. Supply chain management has benefited from the integration of Blockchain and IoT, which improve traceability, transparency, and security across the pharmaceutical supply chain. Regulatory compliance is also becoming more streamlined as companies use advanced technologies to ensure adherence to complex global regulations.
By End-User Analysis
Pharmaceutical companies dominate the Pharma 4.0 Market with a 50% end-user share.
In 2023, Pharmaceutical Companies held a dominant market position in the By End-User segment of the Pharma 4.0 Market, capturing more than a 50% share. Pharmaceutical companies are at the forefront of adopting Pharma 4.0 technologies due to their need to innovate, improve operational efficiency, and maintain competitiveness in a highly regulated industry. These companies invest heavily in AI, IoT, and Big Data to optimize their drug development processes, enhance production capabilities, and ensure quality and compliance across their operations. The adoption of these technologies by pharmaceutical companies is driven by the significant benefits they offer in terms of speed to market, cost reduction, and the ability to manage the increasing complexity of drug development and manufacturing.
Contract Manufacturing Organizations (CMOs) and research institutions also play a vital role in the Pharma 4.0 ecosystem, though they hold smaller shares of the market. CMOs, in particular, are increasingly adopting these technologies to meet the demands of their pharmaceutical clients for high-quality, efficient production services. Research institutions are leveraging Pharma 4.0 technologies, especially AI and Big Data, to accelerate drug discovery services and development, though their market influence remains secondary to that of pharmaceutical companies.
Key Market Segments
By Technology
- IoT
- AI
- Big Data
- Blockchain
- Cloud Computing
- Others
By Application
- Drug Manufacturing
- Quality Control
- Supply Chain Management
- Regulatory Compliance
By End-User
- Pharmaceutical Companies
- Contract Manufacturing Organizations (CMOs)
- Research Institutions
Growth Opportunity
Development of Smart Manufacturing and Quality Control Systems
The development of smart manufacturing and quality control systems presents a significant growth opportunity for the Pharma 4.0 Market in 2024. Smart manufacturing, powered by AI, IoT, and advanced analytics, allows pharmaceutical companies to optimize production processes, reduce waste, and improve product quality. These systems enable real-time monitoring and automated decision-making, ensuring that manufacturing processes are consistently aligned with regulatory standards and quality expectations.
The implementation of smart quality control systems also helps in detecting and correcting errors early in the production cycle, reducing the risk of costly recalls and enhancing overall operational efficiency. As the demand for more efficient and reliable manufacturing processes grows, the adoption of these smart systems is expected to drive significant market growth.
Expansion in Digital Health and Connected Drug Delivery Systems
The expansion of digital health and connected drug delivery systems is another critical opportunity fueling the growth of the Pharma 4.0 Market. Digital health platforms, integrated with Pharma 4.0 technologies, allow for continuous patient monitoring, personalized treatment plans, and enhanced patient engagement. Connected drug delivery systems, such as smart inhalers or wearable injectors, enable precise dosing and real-time tracking of medication adherence, improving patient outcomes.
These innovations not only enhance the effectiveness of treatments but also open new avenues for personalized medicine and patient-centric care. As the healthcare industry increasingly embraces digital transformation, the integration of these advanced systems within the Pharma 4.0 framework will drive innovation and market expansion.
Latest Trends
AI and Machine Learning in Drug Discovery
In 2024, the integration of AI and machine learning in drug discovery and development is expected to be a significant trend driving the Pharma 4.0 Market. AI and machine learning algorithms are transforming the pharmaceutical landscape by enabling faster and more efficient drug discovery processes. These technologies can analyze vast datasets to identify potential drug candidates, predict their efficacy, and optimize clinical trial designs.
By reducing the time and cost associated with bringing new drugs to market, AI and machine learning are not only accelerating innovation but also increasing the success rates of drug development projects. This trend is positioning AI as a cornerstone of Pharma 4.0, where digital transformation meets cutting-edge scientific research.
Digital Twins in Pharmaceutical Production
The use of digital twins for optimizing pharmaceutical production is another key trend shaping the Pharma 4.0 Market in 2024. Digital twins are virtual replicas of physical production processes, enabling real-time monitoring, simulation, and optimization of manufacturing operations. By creating a digital twin of a pharmaceutical production line, companies can predict potential issues, test process changes, and optimize performance without disrupting actual production.
This technology enhances both efficiency and quality control, ensuring that manufacturing processes meet regulatory standards and operate at peak efficiency. The adoption of digital twins is expected to grow as pharmaceutical companies seek to leverage advanced technologies to remain competitive and agile in an increasingly complex market environment.
Regional Analysis
In 2023, Europe led the Pharma 4.0 Market, securing around 41% of the global market share.
This dominance is attributed to the region's strong emphasis on regulatory compliance, innovation in pharmaceutical manufacturing, and the early adoption of advanced digital technologies across the pharmaceutical sector. European countries, particularly Germany, the United Kingdom, and Switzerland, are at the forefront of integrating Industry 4.0 principles into their pharmaceutical production processes, which include the use of artificial intelligence (AI), Internet of Things (IoT), and big data analytics.
Europe's leadership in the Pharma 4.0 Market is further supported by significant investments in research and development (R&D) and the presence of a robust pharmaceutical industry that continuously seeks to enhance efficiency, reduce costs, and ensure product quality. The region's regulatory environment, which strongly supports the digital transformation of the pharmaceutical sector, also plays a crucial role in driving the adoption of Pharma 4.0 technologies.
North America and Asia Pacific are also important players in the Pharma 4.0 Market. North America, driven by the United States, emphasizes innovation and the rapid implementation of new technologies in pharmaceutical manufacturing, with a strong focus on AI and cloud computing.
Key Regions and Countries
North America
- US
- Canada
- Mexico
Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
The global Pharma 4.0 market is undergoing a rapid transformation in 2024, driven by the increasing digitization of pharmaceutical processes. Siemens AG and GE Healthcare are leading the charge by providing cutting-edge automation and digitalization solutions that streamline drug manufacturing, improve quality control, and enhance regulatory compliance. Their innovative technologies are enabling pharmaceutical companies to achieve greater operational efficiency and flexibility.
IBM Corporation and Rockwell Automation, Inc. are at the forefront of integrating artificial intelligence (AI) and machine learning (ML) into Pharma 4.0, allowing for predictive analytics, process optimization, and enhanced decision-making. These technologies are critical for improving drug development timelines and ensuring high standards of quality across the supply chain.
Honeywell International Inc. and Schneider Electric are key players in the automation sector, offering advanced control systems and IoT-enabled devices that ensure real-time monitoring of pharmaceutical production processes. Their solutions are helping pharmaceutical manufacturers reduce downtime and improve productivity.
Meanwhile, Emerson Electric Co. and ABB Ltd. are focusing on robotics and process automation to enhance pharmaceutical production capabilities. Their robotic systems are improving precision, reducing human error, and ensuring compliance with stringent regulatory standards.
Bosch Rexroth AG and Dassault Systèmes are contributing to the market through advanced simulation and digital twin technologies, allowing pharmaceutical companies to simulate production environments and optimize workflows. These innovations are playing a key role in reducing time-to-market for new drugs and ensuring consistent quality in 2024.
Market Key Players
- Siemens AG
- GE Healthcare
- IBM Corporation
- Rockwell Automation, Inc.
- Honeywell International Inc.
- Schneider Electric
- Emerson Electric Co.
- ABB Ltd.
- Bosch Rexroth AG
- Dassault Systèmes
Recent Development
- In February 2024, Siemens AG introduced a new digital twin technology designed to optimize pharmaceutical manufacturing processes. This innovation is expected to reduce production downtime by 20% and improve overall efficiency in drug manufacturing.
- In April 2024, Rockwell Automation, Inc. announced a strategic partnership to integrate AI-driven analytics into Pharma 4.0 systems, enhancing real-time data analysis. This collaboration is projected to improve quality control processes by 25%.
Report Scope
Report Features Description Market Value (2023) USD 3.0 Bn Forecast Revenue (2033) USD 15.2 Bn CAGR (2024-2033) 18.1% Base Year for Estimation 2023 Historic Period 2018-2023 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered By Technology (IoT, AI, Big Data, Blockchain, Cloud Computing, Others), By Application (Drug Manufacturing, Quality Control, Supply Chain Management, Regulatory Compliance), By End-User (Pharmaceutical Companies, Contract Manufacturing Organizations (CMOs), Research Institutions) Regional Analysis North America - The US, Canada, & Mexico; Western Europe - Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe - Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC - China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America - Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa - Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Siemens AG, GE Healthcare, IBM Corporation, Rockwell Automation, Inc., Honeywell International Inc., Schneider Electric, Emerson Electric Co., ABB Ltd., Bosch Rexroth AG, Dassault Systèmes Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Siemens AG
- GE Healthcare
- IBM Corporation
- Rockwell Automation, Inc.
- Honeywell International Inc.
- Schneider Electric
- Emerson Electric Co.
- ABB Ltd.
- Bosch Rexroth AG
- Dassault Systèmes




