Orthopedic Regenerative Surgical Products Market By Product(Viscosupplements, Allografts, Synthetic, Cell-based), By Application (Joint Reconstruction, Orthopedic Pain Management, Trauma Repair, Others), By End-use(Hospitals, Ambulatory Surgical Centers, Others), By Region And Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, And Forecast 2023-2032
-
42418
-
Dec 2023
-
179
-
-
This report was compiled by Correspondence Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Quick Navigation
- Orthopedic Regenerative Surgical Products Market Size, Share, Trends Analysis
- Orthopedic Regenerative Surgical Products Market Dynamics
- Orthopedic Regenerative Surgical Products Market Segmentation Analysis
- Orthopedic Regenerative Surgical Products Industry Segments
- Orthopedic Regenerative Surgical Products Market Growth Opportunity
- Orthopedic Regenerative Surgical Products Market Regional Analysis
- Orthopedic Regenerative Surgical Products Industry By Region
- Orthopedic Regenerative Surgical Products Market Share Analysis
- Orthopedic Regenerative Surgical Products Industry Key Players
- Orthopedic Regenerative Surgical Products Market Recent Development
- Report Scope
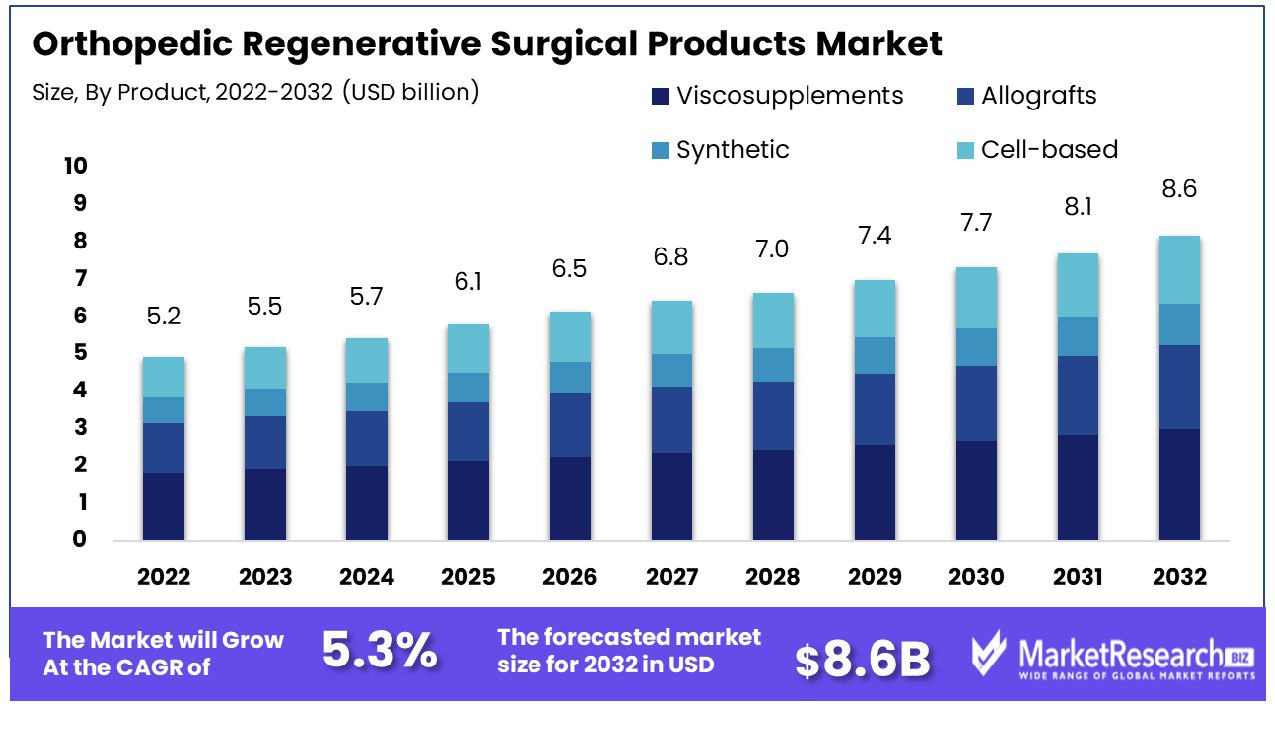
Orthopedic Regenerative Surgical Products Market size is expected to be worth around USD 8.6 Billion by 2032 from USD 5.2 Billion in 2022, growing at a CAGR of 5.3% during the forecast period from 2023 to 2032.
These devices use Bioengineered tissue, biomaterials to tissues, as well as other molecules that permit your body to repair damaged joints and muscles faster. Examples of these products include bone transplants, platelet-rich plasma therapy stem cell therapy, and growth factors which encourage the growth of cells and their differentiation, which results in helping to repair damaged structures.
These products are implemented during orthopedic surgeries to facilitate the regeneration of injured bones, muscles, ligaments, tendons, or cartilage.
Implementation of evidence-based regenerative products is transforming the field - moving from conventional mechanical repair toward biological restoration of musculoskeletal defects caused by trauma or degeneration. Their capacity to "jump start" tissue regeneration is bringing new possibilities for improving outcomes.
Nearly 1 million Americans suffer from orthopedic conditions that limit daily activities. An estimated 988,000 have impairments that impede them from performing basic tasks of living independently; among these are approximately 33,000 children and youth aged 3-21 living with skeletal disabilities in the 2021-2022 academic year alone.
The significant number of affected individuals underscores the demand for innovative skeletal regenerative therapies and surgeries that can enhance outcomes and improve quality of life.
With the rise in skeletal disorders and the need for less invasive treatment and regenerative orthopedic surgery, the regenerative products market is poised for rapid growth. The principal reason for the growth of these markets is the increasing elderly population and the increasing incidence of osteoarthritis and rheumatoid arthritis injuries that result in fractures, ligaments, or other injuries. More than 131 clinical trials conducted since 2006 have sought to better understand the mechanisms and uses of stem cells in medical settings.
Investments in biocompatible materials like PEEK, bioceramics, and porous titanium to improve osteointegration as well as the shift towards customized, patient-specific muscular surgical products are fueling the Orthopedic Regenerative Surgical Products Market growth.
For Instance, ChitogenX is developing an arthroscopic procedure to improve outcomes for 700,000 annual meniscus repair surgeries in the U.S. that currently have a 20-40% failure rate. Supported by a $750,000 grant, ChitogenX will test its proprietary technology on 22 sheep, with surgeries starting in November 2022 and results expected in fall 2023. The CEO highlighted the potential of ChitogenX's versatile platform to enhance regenerative orthopedic surgery.
Improving healthcare expenditure, adoption of advanced surgical tools, and rising awareness regarding regenerative treatments are spurring further industry expansion. Addressing cost constraints and establishing the clinical efficacy of emerging therapies will be vital to future market growth.
Orthopedic Regenerative Surgical Products Market Dynamics
Aging Population Catalyzes Orthopedic Regenerative Surgical Product Demand
The aging global population is a significant driver of the Orthopedic Regenerative Surgical Products Market. As we get older, we become more vulnerable to various orthopedic conditions which include osteoporosis as well as osteoarthritis. This results in more demand for orthopedic surgeries. Regenerative surgical tools that offer innovative solutions for joint and bone repair are especially appealing to those who are old enough and want to keep their mobility intact and enhance their lives.
The growing number of elderly individuals worldwide suggests a sustained increase in the prevalence of age-related orthopedic device conditions, directly impacting the demand for regenerative surgical solutions. This demographic shift is expected to continue, reinforcing the need for advanced muscular regenerative products designed to cater to the unique needs of the aging population.
Chronic Orthopedic Conditions Drive Market Expansion
Chronic orthopedic issues like degenerative bone disorders and sports-related injuries, significantly contribute to the expansion of the Orthopedic Regenerative Surgical Products Market. These ailments typically require long-term care and surgical treatment.
Regenerative surgical products offer innovative treatment options that promote natural healing and recovery, making them a preferred choice for chronic orthopedic conditions. The rising prevalence of these conditions, partly due to lifestyle factors and increased physical activity, necessitates more effective and durable muscular solutions. This trend will likely persist, leading to ongoing market expansion as regenerative products become increasingly integral to managing chronic muscular
Minimally Invasive Procedures Enhance Orthopedic Regenerative Product Adoption
The increasing preference for minimally invasive procedures in orthopedics is driving the growth of the Orthopedic Regenerative Surgical Products Market. Minimally invasive surgeries offer benefits like reduced recovery time and lower risk of complications, appealing to both patients and surgeons. Regenerative surgical products are well-suited for these procedures, as they often require smaller incisions and enable natural tissue regeneration. This compatibility boosts their appeal in the context of modern surgical practices.
As minimally invasive techniques continue to improve and become more widely used, demand for regenerative products that are compatible is anticipated to grow. This indicates an industry that is growing in tandem with the advancements in surgical techniques, and the introduction of regenerative therapies is essential to the progress in minimally-invasive orthopedic procedures.
High Cost of Treatment Limits Growth of Orthopedic Regenerative Surgical Products Market
The high cost associated with orthopedic regenerative surgical products significantly limits their market growth. These modern treatments, which often include advanced materials and cutting-edge technology are usually more costly than traditional orthopedic procedures.
The manufacturing and development costs of these regenerative products along with the special capabilities required for their use can contribute to their expensive cost. This cost factor makes such treatments less accessible to a broader patient base, especially in regions with limited healthcare funding. Consequently, the market for these products is restricted to more affluent demographics or healthcare systems with the capacity to absorb higher costs.
Poor Reimbursement Scenarios Restrain Orthopedic Regenerative Surgical Products Market
Poor reimbursement scenarios further restrain the growth of the orthopedic regenerative surgical products market. Many insurance providers and healthcare systems have yet to fully recognize or cover the costs of these advanced regenerative treatments. This lack of adequate reimbursement policies means that patients often bear a significant portion of the treatment costs.
For many, this financial burden can be prohibitive, leading to a preference for more traditional, covered musculoskeletal treatments. The market's expansion is thus limited by the reluctance of insurance companies and healthcare payers to adapt reimbursement models to include these innovative, more expensive, regenerative surgical options.
Orthopedic Regenerative Surgical Products Market Segmentation Analysis
By Product Analysis
The viscosupplements segment is the leading product in the Orthopedic Regenerative Surgical Products Market. Hyaluronic acid injections are mostly utilized to alleviate knee osteoarthritis discomfort. Their popularity stems from their capability to ease joint pain and improve joint functionality, making them an effective alternative to surgical treatment. Their increasing demand is due to the aging population as well as the increasing prevalence of osteoarthritis. Viscosupplements are also popular due to their security and low incisions compared to surgical procedures.
Allografts segment are used for bone grafting and reconstruction. Synthetic options offer biocompatibility and reduced risk of disease transmission. Cell-based products represent a growing field focusing on tissue regeneration and healing.
By Application Analysis
The joint reconstruction segment remains the most significant application for orthopedic regenerative surgical products. This area encompasses procedures like hip and knee replacements, where such products are vital for enhanced healing and recovery. The application segment's growth is driven by a rising prevalence of joint disorders and innovations in surgical methodologies. Additionally, these advancements contribute to improved patient outcomes and shorter recovery times, leading to increased adoption of regenerative products in orthopedic surgeries.
Orthopedic pain management incorporates products such as viscosupplements for non-surgical relief of pain. Regenerative products are pivotal in trauma repair and cartilage & tendon repair, enhancing the healing process. Other significant applications include spinal surgeries and treatments for sports-related injuries.
By End-use Analysis
Hospitals are the largest end-users of orthopedic regenerative surgical products. Their comprehensive surgical facilities and the capability to handle complex orthopedic procedures drive this segment. Hospitals' investment in advanced technologies and multidisciplinary teams contributes to their dominance.
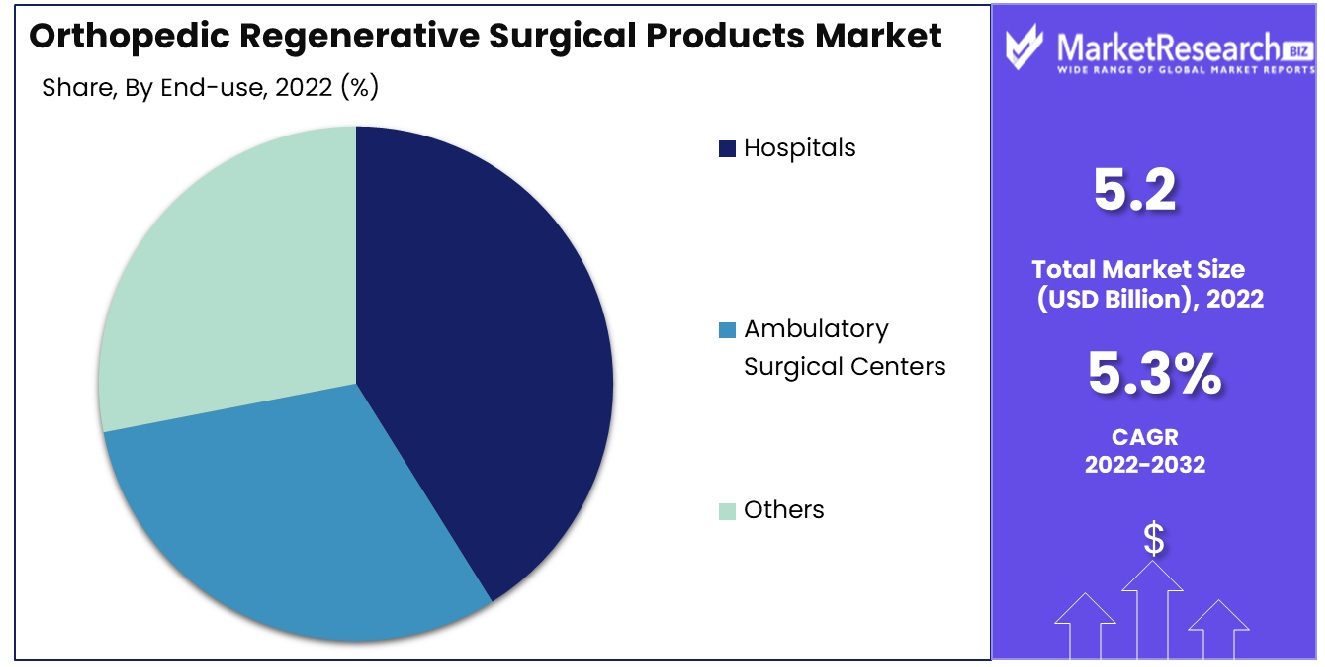
Ambulatory Surgical Centers (ASCs) have gained prominence for outpatient orthopedic procedures, noted for their cost-effectiveness and efficiency in patient care. The 'Others' category encompasses specialty clinics and rehabilitation centers, each playing a crucial role in post-surgical care and long-term patient recovery. The increasing focus on specialized musculoskeletal care and personalized patient management in these settings is contributing to their growing market share.
Orthopedic Regenerative Surgical Products Industry Segments
By Product
- Viscosupplements
- Allografts
- Synthetic
- Cell-based
By Application
- Joint Reconstruction
- Orthopedic Pain Management
- Trauma Repair
- Cartilage & Tendon Repair
- Others
By End-use
- Hospitals
- Ambulatory Surgical Centers
- Others
Orthopedic Regenerative Surgical Products Market Growth Opportunity
Technological Advancements in Biomaterials and Biologics Offer Growth Opportunities in Orthopedic Regenerative Surgical Products Market
The technological advancements in biomaterials and biologics are causing a significant increase in the orthopedic surgical market. Advancements in 3D printing technology and tissue engineering as well as stem cell therapy are changing how musculoskeletal injuries are treated.
These technologies permit the development of more efficient and personalized Regenerative solutions, like customized implant scaffolds and tissues that encourage natural tissue and bone regeneration. The continuous evolution of these technologies is expanding the capabilities of musculoskeletal regenerative products, leading to better patient outcomes and fueling the market growth.
Application Expansion in Degenerative Diseases and Sports Injuries Fuels Market Growth
The expanding application of orthopedic regenerative surgical products in treating degenerative diseases and sports injuries presents a substantial growth opportunity. As the incidence of ailments like osteoarthritis increases with an aging population, and injuries from sports are becoming more frequent and more frequent, the need for advanced treatments for degenerative diseases increases.
Orthopedic regenerative devices are increasingly utilized to repair and rebuild bones and damaged tissues when needed, providing better healing and faster recovery. This widening scope of applications in various orthopedic conditions indicates a strong potential for market expansion, driven by the growing need for effective regenerative treatment options.
Orthopedic Regenerative Surgical Products Market Regional Analysis
North America Dominates with a 40.30% Market Share
North America's significant 40.30% share in the Orthopedic Regenerative Surgical Products Market is primarily driven by its advanced healthcare infrastructure and a strong focus on innovative medical treatments. The region, particularly the United States, is a hub for medical research and development, including regenerative medicine.
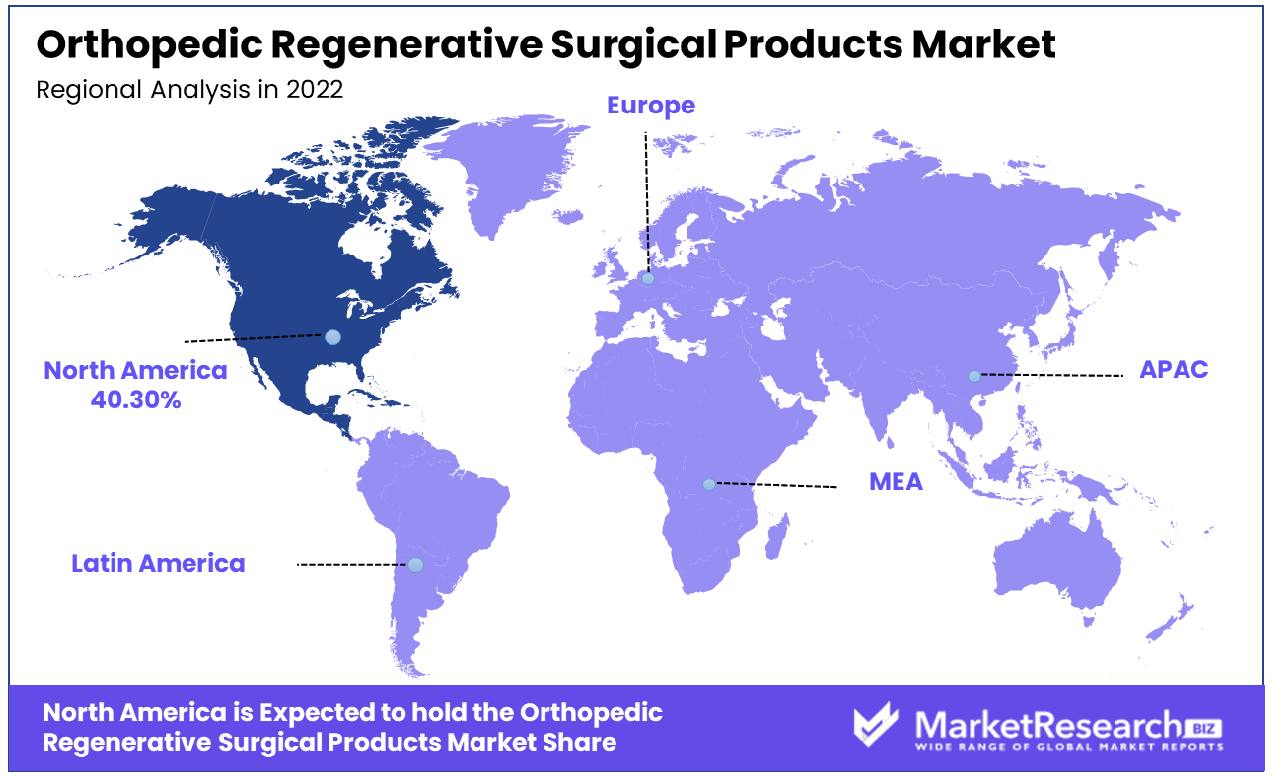
High healthcare expenditure and a growing aging population, which increases the prevalence of musculoskeletal conditions, also contribute to this market dominance. Additionally, the presence of leading medical device companies and a favorable regulatory environment for product approvals boost the market growth in this region.
The market dynamics in North America are influenced by the increasing demand for minimally invasive surgeries and the adoption of advanced regenerative technologies in orthopedics. Moreover, substantial investments in stem cell research and tissue engineering are key factors supporting the development of innovative musculoskeletal regenerative solutions.
Europe's Emphasis on Advanced Healthcare and Research
Europe’s orthopedic regenerative surgical products market benefits from the region's emphasis on advanced healthcare and extensive research in regenerative medicine. The well-established healthcare systems, combined with supportive government policies for healthcare research, foster the development and adoption of innovative orthopedic treatments. The growing aging population in Europe also contributes to the increasing demand for regenerative orthopedic solutions.
Asia-Pacific is a Rapidly Growing Market with Increasing Healthcare Investments
In Asia-Pacific, the orthopedic regenerative surgical products market is experiencing rapid growth. This expansion is driven by increasing healthcare investments, the rising prevalence of orthopedic conditions, and the growing healthcare awareness in emerging economies like China and India. The region's developing healthcare infrastructure and expanding medical tourism industry presents significant growth opportunities for advanced orthopedic treatments, positioning Asia-Pacific as a key emerging market in this sector.
Orthopedic Regenerative Surgical Products Industry By Region
North America
- The US
- Canada
- Rest of North America
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherlands
- Rest of Europe
Asia-Pacific
- China
- Japan
- South Korea
- India
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of Asia Pacific
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of Middle East & Africa
In the Orthopedic Regenerative Surgical Products Market, a sector at the forefront of advanced surgical solutions, the companies listed are key in driving innovation and addressing the evolving needs of musculoskeletal care. Zimmer Biomet and Stryker, as industry leaders, offer a wide range of regenerative products, including bone grafts and tissue regeneration solutions, emphasizing the market's focus on enhancing bone and joint healing processes.
AlloSource and Amniox Medical, Inc., specializing in allografts and amniotic tissues, play crucial roles in advancing regenerative therapies in orthopedics, underscoring the importance of innovative biological materials in surgical recovery. Anika Therapeutics, Inc. and Baxter, known for their medical products, contribute significantly with solutions that support tissue repair and regeneration, reflecting the industry's shift towards minimally invasive and biologically compatible treatments.
Smith & Nephew, Vericel Corporation, and MIMEDX, with their focus on advanced wound care and regenerative medicine, offer technologies and products that are instrumental in improving surgical outcomes in orthopedics. Aptissen S.A and Arthrex, Inc., though smaller in comparison, provide specialized products that cater to specific needs within musculoskeletal surgery, demonstrating the market's diversity and adaptability.
VSY Biotechnology and MiMedx, focusing on biotechnology solutions in orthopedics, showcase the growing importance of biologically derived products in enhancing tissue healing and regeneration. B. Braun Melsungen AG and Integra LifeSciences Corporation, with their broad range of medical devices and surgical solutions, underscore the market's commitment to comprehensive and integrated regenerative solutions.
Collectively, these companies not only drive advancements in the Regenerative Surgical Products Market but also represent a spectrum of strategies, from developing cutting-edge biological materials to providing comprehensive surgical solutions, crucial for advancing the field of regenerative orthopedic surgery.
Orthopedic Regenerative Surgical Products Industry Key Players
- Zimmer Biomet
- Stryker Corporation
- AlloSource
- Amniox Medical, Inc.
- Anika Therapeutics, Inc.
- Smith &Nephew
- Baxter
- Vericel Corporation
- MIMEDX
- Aptissen S.A
- Arthrex, Inc.
- VSY Biotechnology
- MiMedx
- B. Braun Melsungen AG
- Integra LifeSciences Corporation
- OrthoSensor, Inc.
- MiMedx Group, Inc.
Orthopedic Regenerative Surgical Products Market Recent Development
- In July 2022, Anika Therapeutics, Inc. (NASDAQ: ANIK), an early intervention orthopedics organization and leader in joint preservation technology, recently received its final 510(k) approval from the Food and Drug Administration for The IntegrityTM Implant System.
- In July 2022, AlloSource introduced their AlloConnex collection of tendon, ligaments, and fascia products - including an acromioclavicular tendon - AlloSource quadricep tendons may also be utilized with or without bone blocks to perform various surgical procedures; specifically for surgeries on the cruciate ligament.
- In September 2022, Embody, Inc. of Norfolk, Virginia announced they successfully closed a Series C funding round and raised $10.4 million led by Genesis Innovation Group's cultivate(MD) Capital Funds LP--an orthopedic technology venture capital fund dedicated to commercializing healthcare innovations.
- In September 2022, MiMedx of Marietta Georgia, which deals in placental biologics, announced their participation in Innovation Quarter's RegenMed Hub.
Report Scope
Report Features Description Market Value (2022) USD 5.2 Billion Forecast Revenue (2032) USD 8.6 Billion CAGR (2023-2032) 5.3% Base Year for Estimation 2022 Historic Period 2016-2022 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product(viscosupplements, Allografts, Synthetic, Cell-based), By Application (Joint Reconstruction, Orthopedic Pain Management, Trauma Repair, Cartilage and tendon Repair, Others), By End-use(Hospitals, Ambulatory Surgical Centers, Others) Regional Analysis North America - The US, Canada, Rest of North America, Europe - Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe, Asia-Pacific - China, Japan, South Korea, India, New Zealand, Singapore, Thailand, Vietnam, Rest of Asia Pacific, Latin America - Brazil, Mexico, Rest of Latin America, Middle East & Africa - South Africa, Saudi Arabia, UAE, Rest of Middle East & Africa Competitive Landscape Zimmer Biomet, Stryker, AlloSource, Amniox Medical, Inc., Anika Therapeutics, Inc., Smith & Nephew, Baxter, Vericel Corporation, MIMEDX, Aptissen S.A, Arthrex, Inc., VSY Biotechnology, MiMedx, B. Braun Melsungen AG, Integra LifeSciences Corporation, OrthoSensor, Inc., MiMedx Group, Inc., Stryker Corporation Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Zimmer Biomet
- Stryker Corporation
- AlloSource
- Amniox Medical, Inc.
- Anika Therapeutics, Inc.
- Smith &Nephew
- Baxter
- Vericel Corporation
- MIMEDX
- Aptissen S.A
- Arthrex, Inc.
- VSY Biotechnology
- MiMedx
- B. Braun Melsungen AG
- Integra LifeSciences Corporation
- OrthoSensor, Inc.
- MiMedx Group, Inc.




