
Oncolytic Virotherapy Market By Virus Type (Herpes Simplex Virus, Adenovirus, Reovirus, Others), By Application (Melanoma, Breast Cancer, Ovarian Cancer, Others), By End-User (Hospitals, Specialty Clinics, Research Institutes), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
-
50390
-
Aug 2024
-
315
-
-
This report was compiled by Trishita Deb Trishita Deb is an experienced market research and consulting professional with over 7 years of expertise across healthcare, consumer goods, and materials, contributing to over 400 healthcare-related reports. Correspondence Team Lead- Healthcare Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Quick Navigation
Report Overview
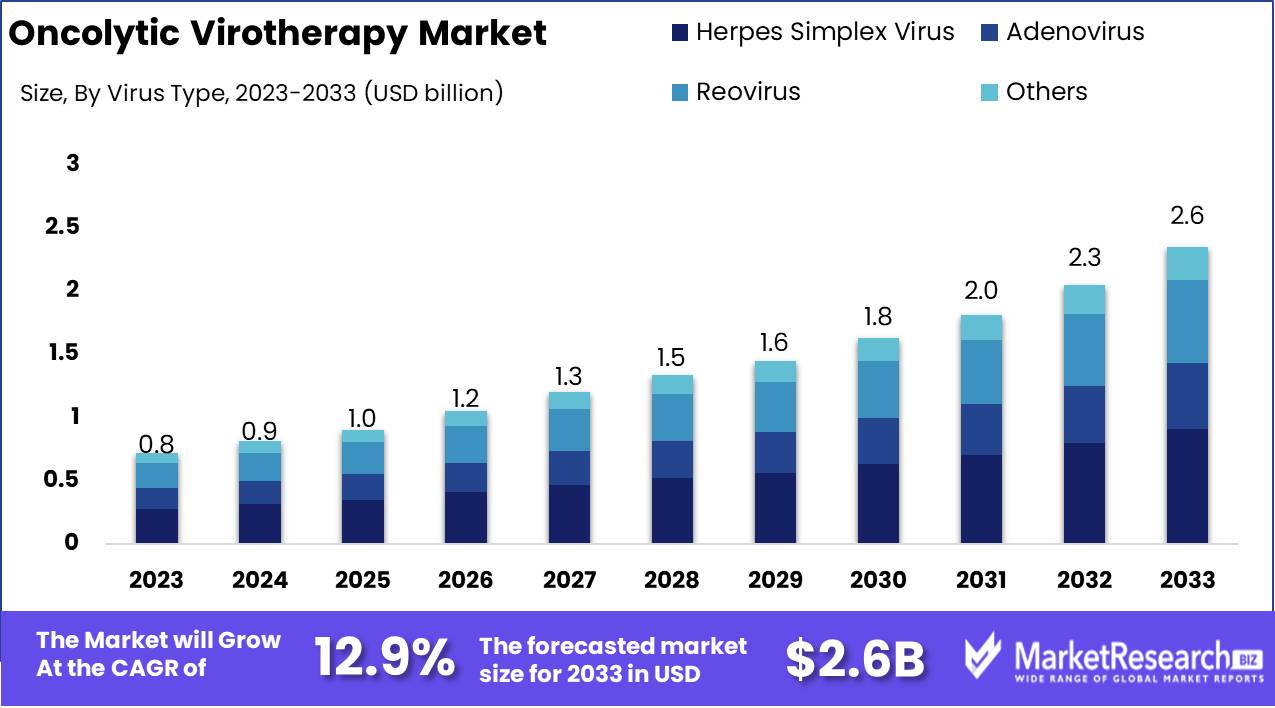
The Global Oncolytic Virotherapy Market was valued at USD 0.8 Bn in 2023. It is expected to reach USD 2.6 Bn by 2033, with a CAGR of 12.9% during the forecast period from 2024 to 2033.
The Oncolytic Virotherapy Market centers on the development and application of oncolytic viruses, which are genetically engineered or naturally occurring viruses that selectively infect and destroy cancer cells while sparing healthy tissue. This innovative approach to personalized cancer treatment leverages the virus’s ability to replicate within tumors, leading to tumor cell lysis and the stimulation of an anti-tumor immune response. As advancements in genetic engineering and clinical research continue, oncolytic virotherapy is emerging as a promising adjunct or alternative to traditional cancer therapies, offering new hope for patients with difficult-to-treat malignancies.
The Oncolytic Virotherapy Market is rapidly gaining traction as a transformative approach in cancer treatment, leveraging the unique ability of viruses to target and destroy cancer cells while simultaneously stimulating the immune system. Recent advancements in the market, particularly through clinical trials utilizing oncolytic herpes simplex virus-1 (HSV-1) and adenovirus-based therapies, have shown significant promise in treating high-grade gliomas (HGGs). These trials indicate the potential to improve median overall survival rates beyond the current 15.6 months for newly diagnosed patients and 6-9 months for those with recurrent cases, marking a critical advancement in the management of these aggressive tumors.
Moreover, the market is witnessing significant innovation with the development of genetically engineered adenoviruses, which have demonstrated remarkable efficacy in preclinical models. These engineered viruses have achieved up to 80% tumor reduction, showcasing their enhanced tumor-targeting capabilities. Such breakthroughs underscore the potential of oncolytic virotherapy to not only improve patient outcomes but also to redefine the therapeutic landscape for various cancers that have proven resistant to conventional treatments.
As the oncolytic virotherapy market continues to evolve, it is poised to play a pivotal role in the future of oncology, offering new therapeutic avenues for patients and driving forward the next generation of cancer treatments. The continued success of clinical trials and preclinical studies will be crucial in translating these innovations into widely accessible treatments, potentially revolutionizing cancer care and offering hope to patients with limited options.
Key Takeaways
- Market Value: The Global Oncolytic Virotherapy Market was valued at USD 0.8 Bn in 2023. It is expected to reach USD 2.6 Bn by 2033, with a CAGR of 12.9% during the forecast period from 2024 to 2033.
- By Virus Type: Herpes Simplex Virus constitutes 35% of the market, used for its effectiveness in targeting cancer cells.
- By Application: Melanoma represents 30%, highlighting the focus on treating this aggressive form of skin cancer.
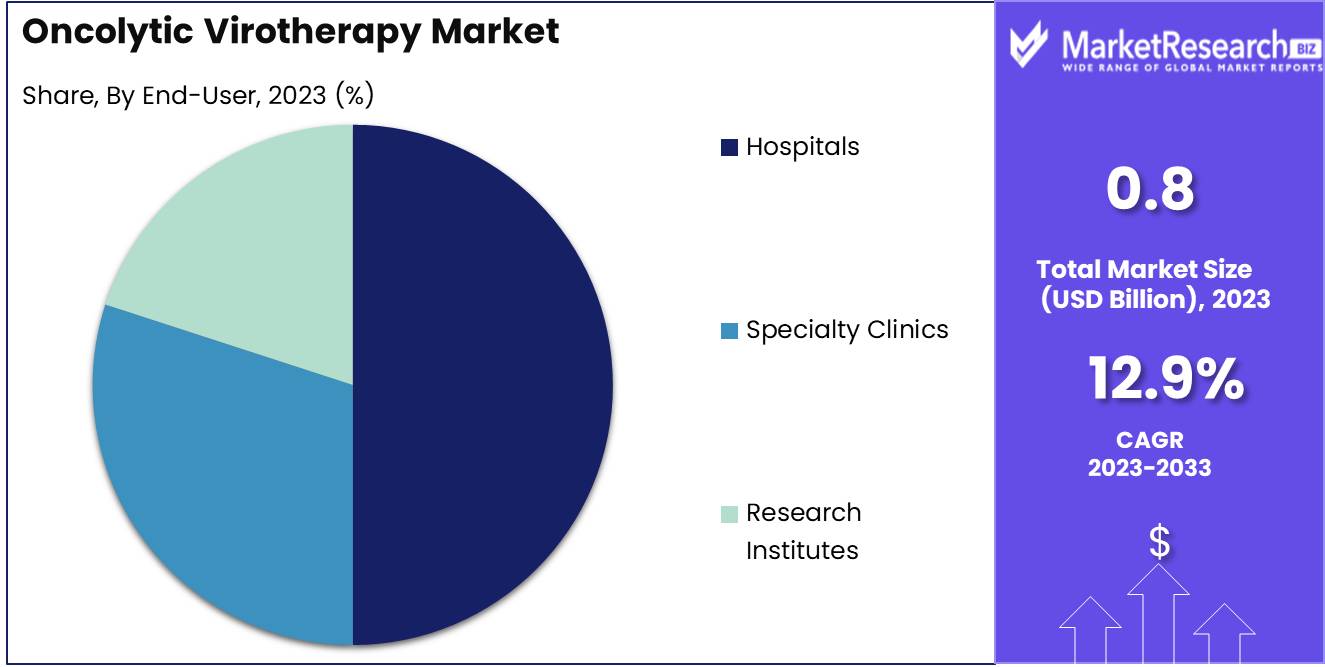
- By End-User: Hospitals account for 50%, utilizing oncolytic virotherapy as part of advanced cancer treatment protocols.
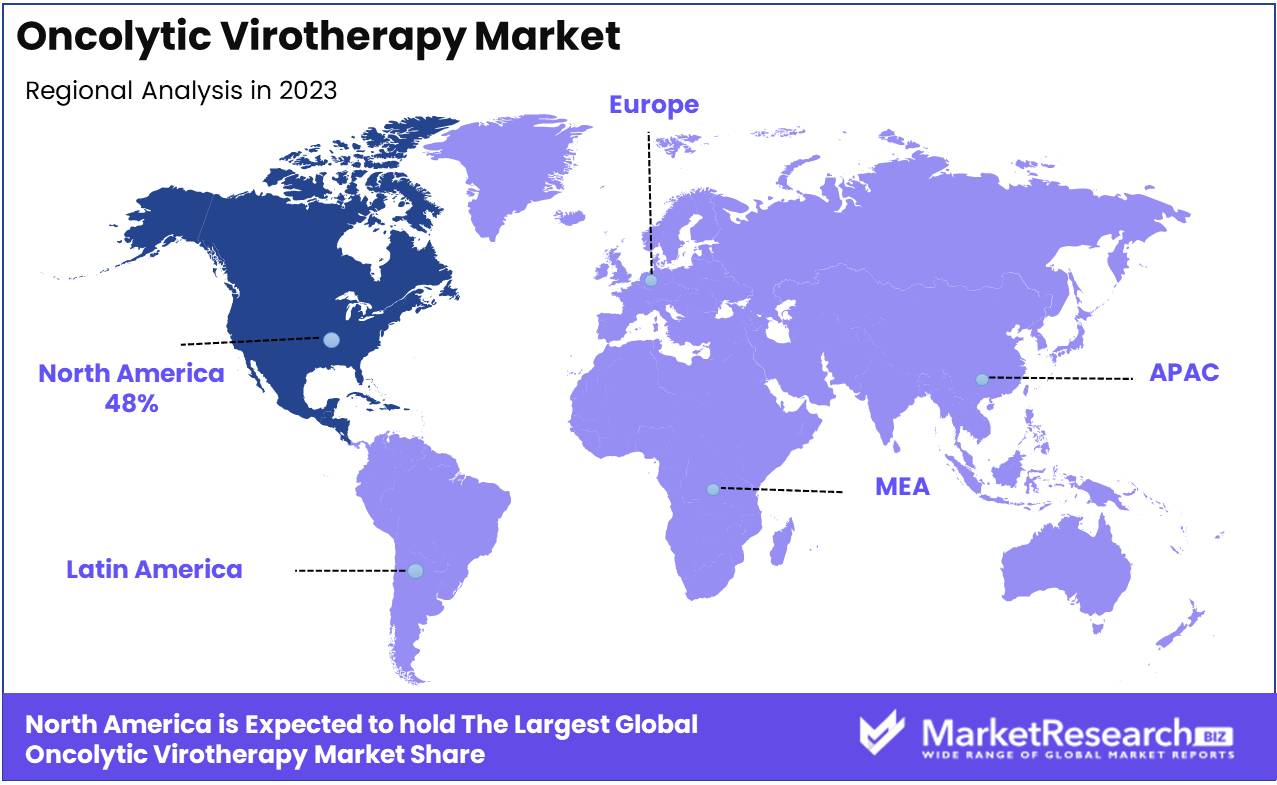
- Regional Dominance: North America holds a 48% market share, driven by cutting-edge cancer research and treatment facilities.
- Growth Opportunity: Expanding clinical trials and research into new cancer types can significantly enhance the oncolytic virotherapy market.
Driving factors
Rising Prevalence of Cancer and Unmet Therapeutic Needs
The rising prevalence of cancer worldwide is one of the most significant driving factors for the growth of the Oncolytic Virotherapy Market. With cancer cases continuing to increase globally, there is a growing demand for innovative therapies that can effectively target and eliminate cancer cells. Despite advancements in conventional treatments like chemotherapy, radiation, and surgery, there remain substantial unmet therapeutic needs, particularly for aggressive or treatment-resistant cancers.
Oncolytic virotherapy, which uses genetically engineered viruses to selectively infect and destroy cancer cells while sparing healthy tissue, offers a promising solution to these unmet needs. As the burden of cancer grows, the demand for oncolytic virotherapy is expected to rise, driving market expansion as healthcare providers seek more effective and targeted treatment options.
Advancements in Genetic Engineering and Virology
Advancements in genetic engineering and virology have been instrumental in the development and refinement of oncolytic viruses, which are central to the growth of the Oncolytic Virotherapy Market. Genetic engineering techniques allow scientists to modify viruses to enhance their ability to target and destroy cancer cells while minimizing potential side effects. These advancements have also enabled the creation of viruses that can stimulate the immune system to recognize and attack cancer cells, a crucial aspect of their therapeutic efficacy.
Progress in virology has deepened our understanding of viral mechanisms and interactions with host cells, leading to the development of more sophisticated and potent oncolytic viruses. The continued evolution of genetic engineering and virology is expected to drive significant innovation in oncolytic virotherapy, making it a more viable and effective option for cancer treatment.
Increasing Focus on Targeted and Immuno-Oncology Therapies
The increasing focus on targeted and immuno-oncology therapies is another key factor contributing to the growth of the Oncolytic Virotherapy Market. Targeted therapies, which aim to specifically attack cancer cells without harming normal cells, and immuno-oncology treatments, which harness the body’s immune system to fight cancer, have revolutionized cancer treatment in recent years. Oncolytic virotherapy fits seamlessly into this paradigm, as it not only targets cancer cells with precision but also triggers an immune response against the tumor.
The dual mechanism of action—direct oncolysis and immune activation—positions oncolytic virotherapy as a complementary or even superior option within the broader landscape of targeted and immuno-oncology therapies. As the oncology field increasingly prioritizes precision medicine and immune-based treatments, the adoption of oncolytic virotherapy is expected to grow, driving market expansion.
Restraining Factors
High Costs of Development and Clinical Trials
One of the primary restraining factors in the Oncolytic Virotherapy Market is the high cost associated with the development and clinical trials of oncolytic viruses. The process of developing oncolytic virotherapies involves extensive research, complex genetic engineering, and rigorous testing to ensure efficacy and safety. These processes require substantial financial investment, often running into hundreds of millions of dollars.
The clinical trials necessary to bring these therapies to market are lengthy and expensive, with multiple phases required to demonstrate safety and efficacy in diverse patient populations. The high costs associated with development and clinical trials can act as a significant barrier to entry for many companies, particularly smaller biotech firms. This financial burden may slow the pace of innovation and limit the availability of new oncolytic virotherapies, thereby restraining market growth.
Regulatory and Safety Challenges
Regulatory and safety challenges present another major restraint in the Oncolytic Virotherapy Market. Oncolytic virotherapies, as novel biological treatments, must navigate complex regulatory pathways to gain approval for clinical use. Regulatory agencies such as the FDA and EMA require extensive safety and efficacy data before approving new therapies, particularly for treatments involving genetically modified organisms like oncolytic viruses. These stringent requirements are necessary to ensure patient safety but can also result in lengthy approval processes and the need for additional studies, further increasing costs and delaying market entry.
The unique nature of oncolytic virotherapy raises specific safety concerns, such as potential off-target effects, immune responses, and the risk of viral shedding. Addressing these challenges requires careful design and monitoring, which adds to the complexity and cost of development. The regulatory and safety hurdles associated with oncolytic virotherapy can slow the introduction of new therapies to the market, ultimately restraining market growth.
By Virus Type Analysis
In 2023, Herpes Simplex Virus held a dominant market position in the By Virus Type segment of the Oncolytic Virotherapy Market, capturing more than a 35% share.
Herpes Simplex Virus (HSV) has emerged as a leading vector in oncolytic virotherapy due to its ability to selectively replicate in tumor cells while sparing normal tissues. HSV-based therapies, such as T-VEC (Talimogene laherparepvec), have shown promising results in clinical trials, particularly for treating melanoma. The virus's efficacy in stimulating the immune system to recognize and attack cancer cells has contributed to its dominant position in the market. The engineering flexibility of HSV, allowing for the insertion of therapeutic genes, further enhances its therapeutic potential, making it the virus of choice for many ongoing research and clinical applications.
Adenovirus and Reovirus are also significant players in the oncolytic virotherapy landscape. Adenovirus, known for its ability to infect a wide range of cell types, is utilized in several clinical trials, particularly for cancers that are less responsive to traditional treatments. Reovirus, which preferentially replicates in Ras-activated tumor cells, is gaining attention for its potential in treating various solid tumors. However, the clinical success and commercial availability of HSV-based therapies keep it ahead of these other virus types in the oncolytic virotherapy market.
By Application Analysis
In 2023, Melanoma held a dominant market position in the By Application segment of the Oncolytic Virotherapy Market, capturing more than a 30% share.
Melanoma has become a primary focus for oncolytic virotherapy, largely due to the success of clinical trials demonstrating the efficacy of oncolytic viruses in treating this aggressive form of skin cancer. The ability of oncolytic virotherapy to not only directly destroy melanoma cells but also to enhance the immune system's ability to target and eliminate residual cancer cells has positioned this therapy as a critical advancement in melanoma treatment. The FDA approval of T-VEC, the first oncolytic virus approved for melanoma treatment, has further solidified the application of virotherapy in this area, driving its dominant market share.
Other applications such as Breast Cancer and Ovarian Cancer are also being explored with promising early-stage results. Oncolytic virotherapy's potential to be combined with other treatments, such as immune checkpoint inhibitors, is expanding its use beyond melanoma. However, the extensive clinical data supporting its use in melanoma, along with its regulatory approval, ensures that this application remains the largest segment within the market.
By End-User Analysis
In 2023, Hospitals held a dominant market position in the By End-User segment of the Oncolytic Virotherapy Market, capturing more than a 50% share.
Hospitals are the primary end-users of oncolytic virotherapy, given their role in administering complex cancer treatments and managing patient care. The adoption of oncolytic virotherapy in hospitals has been driven by the growing number of clinical trials and approved therapies available for cancer treatment. Hospitals' access to advanced medical infrastructure and multidisciplinary teams capable of managing the intricacies of oncolytic virotherapy ensures that they are well-positioned to offer these cutting-edge treatments to patients, thus maintaining their dominance in the market.
Specialty Clinics and Research Institutes also contribute to the market, particularly in niche areas of cancer treatment and early-stage clinical research. Specialty Clinics focus on providing advanced cancer care, often incorporating new therapies like oncolytic virotherapy into their treatment regimens. Research Institutes play a critical role in advancing the development of oncolytic viruses and conducting the clinical trials necessary for their approval. However, the broader patient base and comprehensive care capabilities of hospitals place them at the forefront of the oncolytic virotherapy market.
Key Market Segments
By Virus Type
- Herpes Simplex Virus
- Adenovirus
- Reovirus
- Others
By Application
- Melanoma
- Breast Cancer
- Ovarian Cancer
- Others
By End-User
- Hospitals
- Specialty Clinics
- Research Institutes
Growth Opportunity
Development of Novel Oncolytic Viruses with Enhanced Efficacy
One of the most promising opportunities in the Oncolytic Virotherapy Market for 2024 is the development of novel oncolytic viruses with enhanced efficacy. Advances in genetic engineering and virology have enabled the creation of oncolytic viruses that are more potent and selective in targeting cancer cells, while also minimizing potential side effects. These next-generation viruses are being designed to overcome resistance mechanisms within tumors, increasing their therapeutic effectiveness.
Researchers are developing oncolytic viruses that can be armed with genes encoding therapeutic proteins, further enhancing their ability to stimulate anti-tumor immune responses. The development of these innovative oncolytic viruses offers significant potential for improving cancer treatment outcomes, particularly in patients with hard-to-treat tumors, and is expected to drive substantial growth in the market.
Expansion in Combination Therapies with Immune Checkpoint Inhibitors
Another key growth opportunity in 2024 is the expansion of oncolytic virotherapy in combination with immune checkpoint inhibitors. Immune checkpoint inhibitors have revolutionized cancer treatment by enabling the immune system to recognize and attack cancer cells more effectively. However, not all patients respond to these therapies, and combining them with oncolytic virotherapy has shown promise in enhancing treatment efficacy. Oncolytic viruses can modulate the tumor microenvironment, making it more susceptible to immune attack and increasing the effectiveness of checkpoint inhibitors.
This combination approach is gaining traction in clinical trials, with several studies demonstrating improved patient outcomes compared to monotherapies. As more data supporting the synergy between oncolytic viruses and immune checkpoint inhibitors emerges, this strategy is likely to become a cornerstone of cancer therapy, offering significant opportunities for market expansion.
Latest Trends
Use of Personalized Oncolytic Virotherapy Based on Tumor Profiling
In 2024, the trend toward personalized oncolytic virotherapy is set to gain significant momentum in the global market. With advances in tumor profiling technologies, it is now possible to analyze the genetic and molecular characteristics of individual tumors in greater detail. This information allows researchers and clinicians to design personalized oncolytic viruses that are specifically tailored to the unique attributes of a patient’s tumor.
By matching the oncolytic virus to the tumor’s specific vulnerabilities, the efficacy of the treatment can be significantly enhanced, leading to better patient outcomes. This personalized approach is expected to drive increased adoption of oncolytic virotherapy, particularly in cases where standard treatments have failed. As precision medicine continues to evolve, personalized oncolytic virotherapy will likely become a standard component of cancer treatment protocols, offering new hope for patients with challenging tumors.
Growing Interest in Oncolytic Viruses for Solid Tumors
Another major trend in 2024 is the growing interest in using oncolytic viruses to target solid tumors. While oncolytic virotherapy has shown promise in treating hematologic malignancies, its application to solid tumors has historically been more challenging due to factors such as the tumor microenvironment and immune evasion. However, recent advancements in viral engineering and delivery methods are overcoming these barriers, leading to renewed interest in applying oncolytic virotherapy to a wider range of solid tumors.
Clinical trials are increasingly focusing on solid tumors such as glioblastoma, melanoma, and pancreatic cancer, where oncolytic viruses are being tested both as monotherapies and in combination with other treatments. This growing interest is expected to significantly expand the market, as oncolytic virotherapy becomes a viable option for treating some of the most difficult-to-treat cancers.
Regional Analysis
North America is the dominating region in the Oncolytic Virotherapy Market, holding a commanding 48% share.
In North America, the Oncolytic Virotherapy market is thriving, driven by advanced healthcare infrastructure, significant investment in cancer research, and strong support from government agencies such as the FDA. The United States is at the forefront of this market, with numerous clinical trials and research initiatives focused on developing novel oncolytic virotherapy treatments. The presence of leading biopharmaceutical companies and research institutions further solidifies North America's dominance in this market, contributing to its 48% market share. Additionally, favorable regulatory pathways and high healthcare expenditure are accelerating the adoption of oncolytic virotherapy in the region.
Europe is another critical region in the Oncolytic Virotherapy Market, with countries like Germany, the United Kingdom, and France leading the charge. The region benefits from a strong focus on cancer research, supported by collaborations between academic institutions and biopharma companies. European governments have also been proactive in funding oncolytic virotherapy research, enhancing the region’s market potential. The increasing prevalence of cancer and the growing emphasis on innovative cancer treatments are key drivers of market growth in Europe, making it a significant player in the global market.
The Asia Pacific region is witnessing rapid growth in the Oncolytic Virotherapy Market, driven by increasing healthcare investments and a growing focus on cancer treatment. Countries such as China, Japan, and South Korea are leading the market, with substantial research and development efforts in oncolytic virotherapy. The region's large patient population and rising incidence of cancer are key factors contributing to market expansion.
Key Regions and Countries
North America
- US
- Canada
- Mexico
Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
The global Oncolytic Virotherapy Market in 2024 is significantly influenced by key companies including Amgen Inc., Oncolytics Biotech Inc., SillaJen Inc., PsiOxus Therapeutics Ltd, VCN Biosciences, Genelux Corporation, Oncorus Inc., Vyriad Inc., Targovax ASA, and Lokon Pharma AB.
Amgen Inc. and Oncolytics Biotech Inc. are pivotal in advancing oncolytic virotherapy, leveraging their robust research and development capabilities to drive innovation in viral-based cancer treatments. Amgen’s extensive expertise in oncology and Oncolytics Biotech’s focused virotherapy approaches are central to market developments.
SillaJen Inc. and PsiOxus Therapeutics Ltd. contribute through their proprietary oncolytic viruses, targeting specific cancer types and enhancing therapeutic efficacy. VCN Biosciences and Genelux Corporation focus on developing novel virotherapy solutions with improved safety and efficacy profiles.
Oncorus Inc. and Vyriad Inc. are notable for their advancements in oncolytic virus engineering, optimizing viral vectors for enhanced cancer targeting. Targovax ASA and Lokon Pharma AB offer innovative approaches in virotherapy, contributing to the expanding treatment options for cancer patients.
Market Key Players
- Amgen Inc.
- Oncolytics Biotech Inc.
- SillaJen Inc.
- PsiOxus Therapeutics Ltd
- VCN Biosciences
- Genelux Corporation
- Oncorus Inc.
- Vyriad Inc.
- Targovax ASA
- Lokon Pharma AB
Recent Development
- In May 2024, VCN Biosciences launched a collaboration with a major pharmaceutical company to develop next-generation oncolytic virotherapy. This partnership aims to enhance the efficacy of treatments for hard-to-treat cancers by 25%.
- In March 2024, Oncolytics Biotech Inc. secured $75 million in funding to advance its lead oncolytic virus therapy in late-stage clinical trials. This funding will support the company's efforts to bring new treatments to the market.
Report Scope
Report Features Description Market Value (2023) USD 0.8 Bn Forecast Revenue (2033) USD 2.6 Bn CAGR (2024-2033) 12.9% Base Year for Estimation 2023 Historic Period 2018-2023 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered By Virus Type (Herpes Simplex Virus, Adenovirus, Reovirus, Others), By Application (Melanoma, Breast Cancer, Ovarian Cancer, Others), By End-User (Hospitals, Specialty Clinics, Research Institutes) Regional Analysis North America - The US, Canada, & Mexico; Western Europe - Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe - Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC - China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America - Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa - Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Amgen Inc., Oncolytics Biotech Inc., SillaJen Inc., PsiOxus Therapeutics Ltd, VCN Biosciences, Genelux Corporation, Oncorus Inc., Vyriad Inc., Targovax ASA, Lokon Pharma AB Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Amgen Inc.
- Oncolytics Biotech Inc.
- SillaJen Inc.
- PsiOxus Therapeutics Ltd
- VCN Biosciences
- Genelux Corporation
- Oncorus Inc.
- Vyriad Inc.
- Targovax ASA
- Lokon Pharma AB




