Global Generative AI in Clinical Trials Market By Application (Data generation, Clinical trial design, and Other ), By Technology (Variational Autoencoders (VAEs), Generative Adversarial Networks (GANs), and Other ), By End-Use, By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, and Forecast 2024-2033
-
38040
-
April 2024
-
300
-
-
This report was compiled by Vishwa Gaul Vishwa is an experienced market research and consulting professional with over 8 years of expertise in the ICT industry, contributing to over 700 reports across telecommunications, software, hardware, and digital solutions. Correspondence Team Lead- ICT Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Quick Navigation
Report Overview
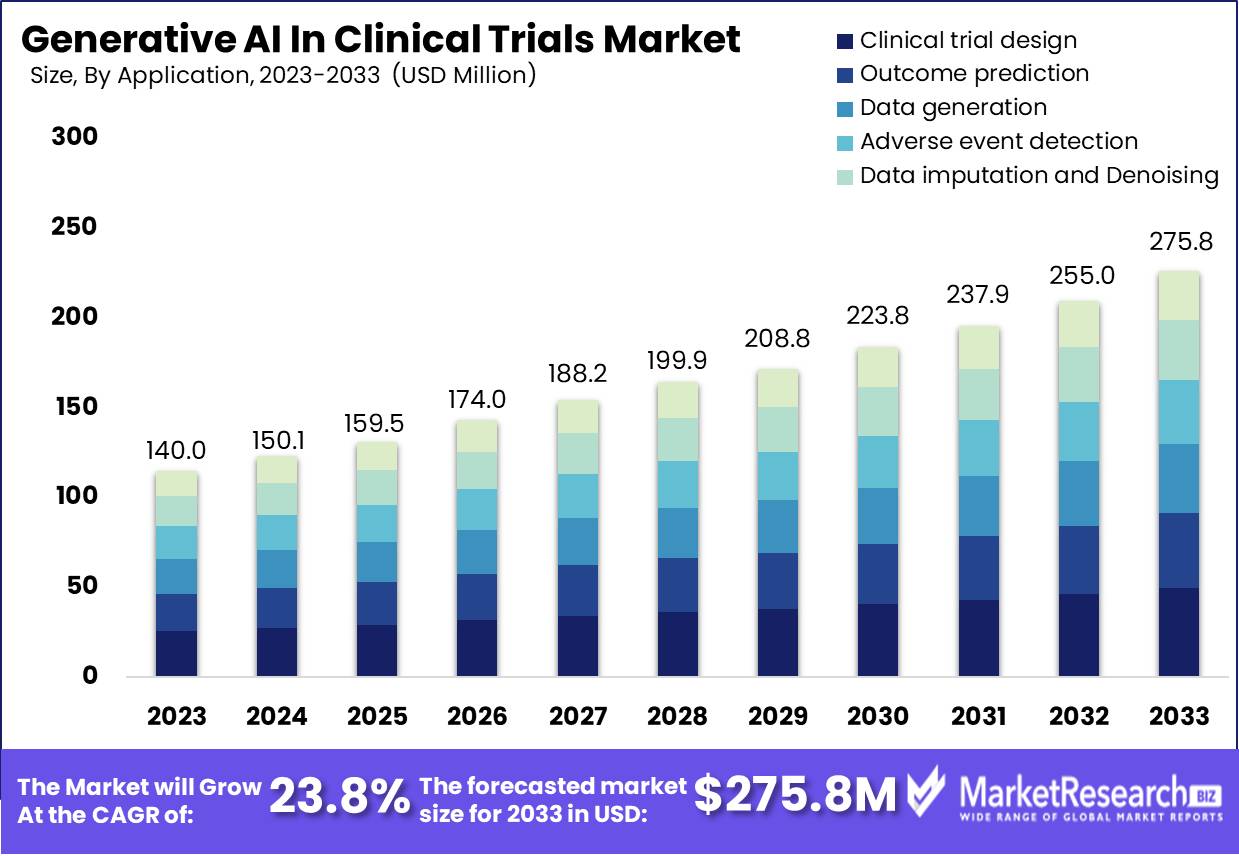
Global Generative AI in Clinical Trials Market size is expected to be around USD 275.8 million by 2033 from USD 140 million in 2023, growing at a CAGR of 23.8%, during the forecast period from 2024 to 2033.
The surge in demand for cloud-based applications and solutions and high-end requirements in pharmaceutical and medical centers are some of the main key driving factors for generative AI in the clinical trials market. In the last few decades, the accessibility to patient medical information has doubled with quick progression in data analytics applications and technologies that have substantially changed many sectors of medicine, starting from the initial stage of drug discovery and study to patient treatment. One of the important applications of new technology is in progress to rationalize and advance clinical research.
According to an article published by Medium in October 2023, states that with the investment, Insilico Medicine is developing a drug that is fully generated by AI to treat patients suffering from idiopathic pulmonary fibrosis. This lung-scarring chronic disease has currently affected 100,000 individuals in the US, as per the National Institute of Health. Currently, the first AI-developed drug has gone into phase 2 of clinical trials as per the report generated by Forbes in June 2023.
Advanced technologies such as generative AI provide the potential to resolve many challenging areas of drug development which has several benefits for the pharmaceutical industries, patients, payers, and investigators. GenAI-based study model helps to enhance and fasten the development of patient-centric models. This helps to decrease patient burden, boosts the likelihood of success, and also augments overall efficacy. Such modern technology has various innovative ways of gathering clinical trial information and lessening dependency on in-person trial sites. For example, by collecting information from body sensors and wearable tools such as bracelets, heart monitors, and sensor-based clothing, clinical researchers can supervise patient’s vital signs and other required information remotely. GenAI algorithms, in integration with the latest wearable technology, can disclose actual-time insights into study implementation and patient adherence.
There are several advantages of using generative AI in clinical medical trials GenAI automates the medical document review by identifying human errors and eliminating them, it also boosts the protocol models, with the help of electronic health records which is known as EHR, powered by GenAI helps to analyze patient’s demographics and site performance history, it also provides actual-time safety monitoring with digital twin models which helps the researchers to understand the ongoing health condition data, past medical history and genetic. The demand for generative AI in clinical trials will rapidly increase due to its requirement in different fields of medical science that will help in market expansion during the forecasted period.
Key Takeaways
- Market Growth: Global Generative AI in Clinical Trials Market size is expected to be around USD 275.8 million by 2033 from USD 140 million in 2023, growing at a CAGR of 23.8%, during the forecast period from 2024 to 2033.
- Based on Application: Generative AI dominates Clinical Trial Design, capturing over 45% market share, and enhancing efficiency and precision in healthcare research.
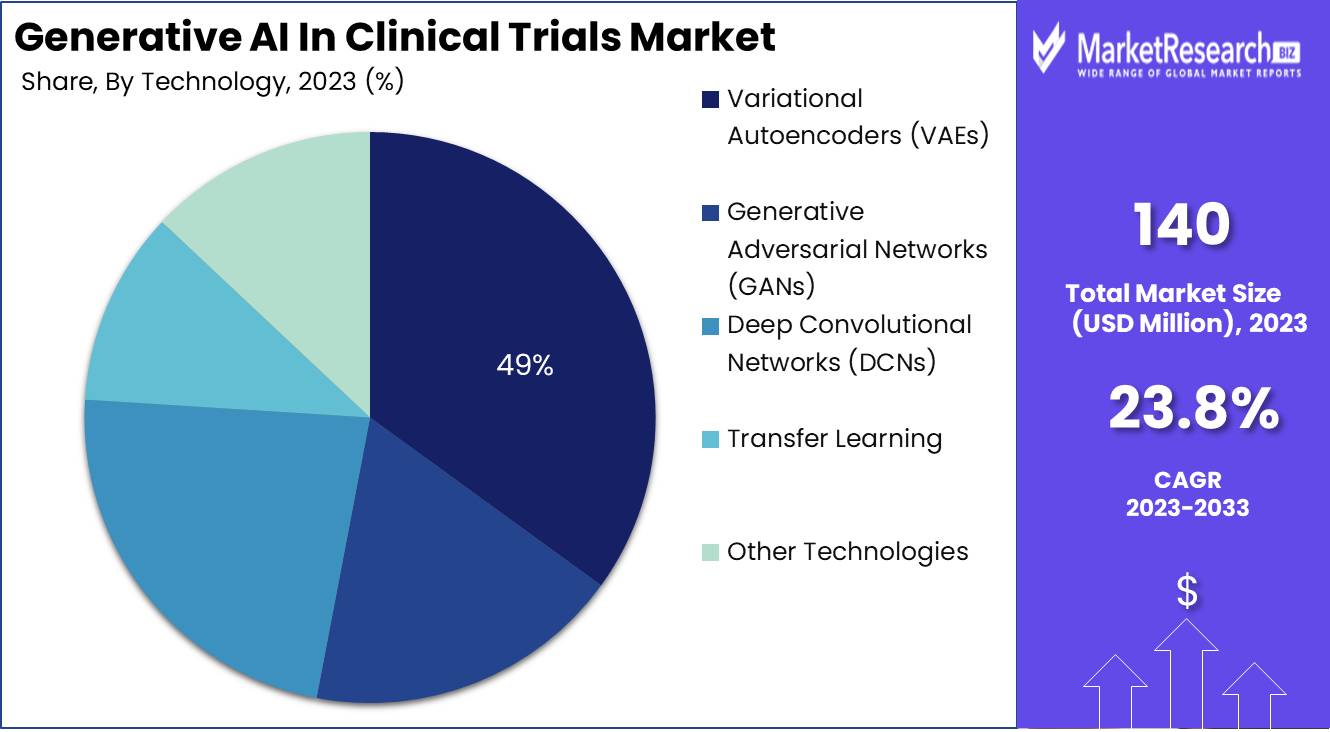
- Based on Technology: In 2023, Variational Autoencoders (VAEs) led with 49% market share, revolutionizing clinical trials with synthetic patient data.
- Based on End-Use: In 2023, researchers led the generative AI clinical trials market, revolutionizing trial design and analysis.
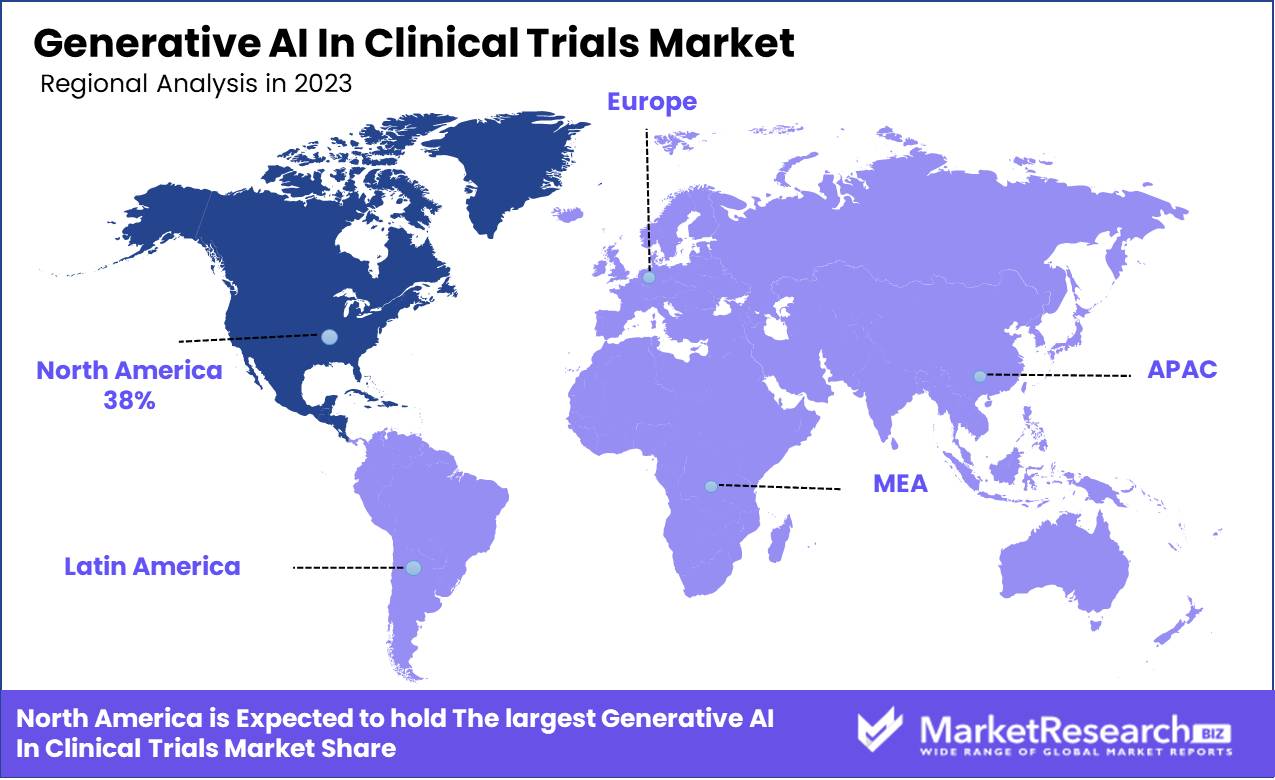
- Regional Dominance: North America Dominates with 38% Market Share in Generative AI In Clinical Trials Market.
- Growth Opportunity: Generative AI is crucial for faster, safer, and more efficient drug development in the global pharmaceutical industry.
Driving Factors
Enhancing Drug Discovery and Development Through AI-Driven Innovation
The increasing complexity and costs associated with traditional drug discovery methods are pivotal drivers for the adoption of generative AI. This technology leverages deep learning algorithms to predict molecular behavior, optimize drug formulations, and simulate clinical outcomes, thus significantly reducing the time and financial investment required to bring a new drug to market. For instance, AI can analyze vast datasets to identify potential drug candidates with higher precision and at speeds unattainable by human researchers alone. This capability not only streamlines the discovery process but also enhances the predictability of drug efficacy and safety profiles, thereby reducing the risk of costly failures in later stages.
Synthetic Data Generation: Expanding the Frontiers of Clinical Research
Generative AI plays a critical role in the creation of synthetic data for clinical trials. This data, which is artificially generated to mimic real patient data, is crucial in situations where actual clinical data is scarce or difficult to obtain, such as in rare diseases. Synthetic data can improve the design and robustness of clinical trials by providing a larger and more diverse dataset for preliminary analyses, ensuring that trials are well-informed before they commence. Moreover, this technology adheres to privacy regulations by offering an alternative to the use of sensitive real patient data, thereby navigating ethical concerns and regulatory compliance more smoothly.
Optimizing Patient Recruitment: Accelerating Trial Timelines and Enhancing Data Quality
Patient recruitment is one of the most challenging aspects of clinical trials, often causing delays and increased costs. Generative AI enhances this process by predicting the best candidate profiles for a given trial, and analyzing existing electronic health records to identify potential participants more rapidly. This optimization not only accelerates the recruitment phase but also ensures a higher quality of data, as participants are more accurately matched to the trial's requirements. AI-driven tools can also forecast patient retention rates and potential drop-out points, allowing trial coordinators to implement preventative strategies, thus maintaining the integrity and momentum of the trial.
Restraining Factors
Data Privacy and Security: A Critical Gatekeeper in the Expansion of Generative AI in Clinical Trials
In the rapidly evolving domain of generative AI applications in clinical trials, data privacy and security emerge as paramount concerns. These factors act as both gatekeepers and enablers in the market's expansion. Clinical trials involve sensitive patient data that is highly regulated under laws such as the General Data Protection Regulation (GDPR) in Europe and the Health Insurance Portability and Accountability Act (HIPAA) in the United States. The application of generative AI in this field requires handling and processing large volumes of data, raising significant concerns about the privacy and security of patient information.
The fear of data breaches and the potential misuse of personal health information can hinder the adoption of AI technologies. Healthcare providers and patients alike seek assurance that new technologies will not compromise their data. As a result, AI developers must invest heavily in secure data practices and technologies, such as advanced encryption methods and robust data anonymization techniques, which can increase the cost and complexity of AI solutions. However, these investments are critical as they not only comply with stringent data protection laws but also build trust with stakeholders, thereby facilitating wider acceptance and integration of AI in clinical trials.
Regulatory Compliance: Steering the Course of Generative AI Deployment
The intersection of generative AI and regulatory compliance is a dynamic area of concern that significantly impacts the growth of AI in clinical trials. Regulatory bodies around the world are still grappling with the implications of AI and often operate under frameworks that do not fully address the novel challenges posed by these technologies. For instance, the U.S. Food and Drug Administration (FDA) has been actively working to create a more flexible regulatory framework that adapts to the rapid advancements in artificial intelligence, yet the current guidelines remain a work in progress.
Compliance with existing and evolving regulations is not only a legal requirement but also a critical factor in ensuring the efficacy and safety of clinical trials incorporating Artificial Intelligence. The need for continuous updates in regulatory policies to keep pace with technological advancements means that AI developers must remain agile and proactive in compliance matters. This ongoing adaptation can slow down the implementation process, as developers need to ensure that their AI solutions can withstand regulatory scrutiny and meet all applicable standards.
By Application Analysis
Generative AI dominates Clinical Trial Design, capturing over 45% market share, and enhancing efficiency and precision in healthcare research.
In 2023, the Generative AI in Clinical Trials Market exhibited a diverse application landscape, with several key segments driving innovation and efficiency in trial design and management. The segments of this market include Data Generation, Clinical Trial Design, Outcome Prediction, Adverse Event Detection, Data Imputation and Denoising, and Other Applications.
Among these, Clinical Trial Design held a dominant market position, capturing more than a 45% share in the Based on Application segment. This prominence underscores the critical role of generative AI in enhancing the strategic planning and execution phases of clinical trials. By leveraging advanced algorithms, this technology facilitates the creation of more accurate and robust trial designs, enabling researchers to simulate numerous trial scenarios and predict their outcomes before actual implementation. This capability significantly reduces the time and cost associated with clinical trials by identifying potential hurdles and efficacy issues at an early stage.
The substantial share held by the Clinical Trial Design segment indicates a growing reliance on AI-driven methodologies to streamline research protocols and improve the precision of health outcomes. As the market continues to evolve, it is expected that the integration of AI in other segments, such as Data Generation and Outcome Prediction, will also see substantial growth, driven by the ongoing need for efficiency and innovation in clinical research.
This trend points to broader adoption of AI technologies in the healthcare sector, suggesting that future market strategies should focus on enhancing AI capabilities and expanding their applications to fully exploit the potential of AI in revolutionizing clinical trials.
By Technology Analysis
In 2023, Variational Autoencoders (VAEs) led with a 49% market share, revolutionizing clinical trials with synthetic patient data.
In 2023, Variational Autoencoders (VAEs) emerged as leaders within the technology segment of the Generative AI in Clinical Trials Market, commanding over 49% of the market share. VAEs are particularly influential in reshaping clinical trial methodologies, notably in drug discovery and the simulation of patient data. Their capacity to create diverse, high-quality synthetic data that mirrors real patient data significantly enhances both the efficacy and dependability of clinical trials. This dominant position highlights the critical role VAEs play in optimizing research processes through advanced data-handling capabilities.
Adjacent to VAEs in market presence are Generative Adversarial Networks (GANs) and Deep Convolutional Networks (DCNs), each vital for scenarios requiring intricate visual data, such as dermatological and radiographic trials. Meanwhile, Transfer Learning is gaining momentum by allowing the reuse of models across various datasets, significantly cutting development costs and time. As these AI technologies continue to advance rapidly, the field is poised for further evolution, prompting stakeholders in clinical trials to remain vigilant and proactive in integrating these innovative tools to maintain competitiveness and enhance operational efficiencies.
By End-Use Analysis
In 2023, researchers led the generative AI clinical trials market, revolutionizing trial design and analysis.
In 2023, researchers and scientists maintained a dominant position in the generative AI in clinical trials market within the end-use segment, due to their critical role in leveraging AI to enhance clinical trial processes. Generative AI has transformed their approach to protocol development and patient selection, allowing for the generation of synthetic data and simulations of patient responses that predict trial outcomes more accurately. This advancement reduces costs and time, improves trial reliability, and enables the creation of targeted treatments for complex diseases through more personalized medicine approaches.
The integration of generative AI has significantly improved data management and analytics, accelerating the pace of research and elevating data quality. This leads to more definitive trial results and the ability to design trials that are more inclusive of diverse patient populations. The continued use of AI by researchers is expected to persist as a key driver of innovation and efficiency in clinical trials, showcasing the substantial impact of AI technologies in refining and advancing clinical research methodologies.
Key Market Segments
Based on Application
- Data generation
- Clinical trial design
- Outcome prediction
- Adverse event detection
- Data imputation and Denoising
- Other Applications
Based on Technology
- Variational Autoencoders (VAEs)
- Generative Adversarial Networks (GANs)
- Deep Convolutional Networks (DCNs)
- Transfer Learning
- Other Technologies
Based on End-Use
- Researchers and Scientists
- Healthcare Professionals
- Clinical Trial Sponsors and CROs
- Data Analysts and Biostatisticians
- Other End Uses
Growth Opportunity
Market Size Expansion: Pioneering New Frontiers
The generative AI sector in clinical trials is poised for significant expansion. This technology is revolutionizing the way clinical trials are designed, executed, and analyzed, offering unparalleled efficiencies in developing medical treatments.
The integration of AI allows for the simulation of patient responses and the generation of synthetic data, which accelerates the trial phases and reduces costs. With a growing emphasis on precision medicine and the rising demand for faster drug development cycles, generative AI is set to expand its market size dramatically. Industry leaders and investors are advised to capitalize on this trend by fostering AI-driven innovations and partnerships that enhance trial efficacy and patient safety.
Global Pharmaceutical Market Integration: A Symbiotic Relationship
Generative AI's integration into the global pharmaceutical market represents a transformative shift towards more agile and data-driven research environments. As pharmaceutical giants seek to reduce the time-to-market for new therapies, AI tools are becoming essential in streamlining research processes and enhancing decision-making capabilities.
The ability of generative AI to predict drug interactions and treatment outcomes earlier in the drug development process enables more strategic allocation of resources and better management of trial risks. For stakeholders, the integration of generative AI into their operations is not just an opportunity—it is becoming a necessity to maintain a competitive advantage in a rapidly evolving industry.
Latest Trends
Increased Funding for AI-Driven Drug Discovery
The Generative AI in clinical trials market is witnessing a significant uptick in funding, primarily driven by its potential to revolutionize drug discovery processes. This infusion of capital is not just from traditional biopharmaceutical investors but increasingly from technology-focused venture capital firms. The rationale behind this trend is the transformative efficiency that AI brings to drug development—reducing time frames from discovery to trials, thereby slashing costs and enhancing the potential for personalized medicine solutions. As these technologies prove their value, we anticipate a continued surge in funding, which in turn accelerates innovation and adoption across the sector.
Predictive Maintenance in Manufacturing
Another emerging trend is the integration of generative AI in clinical trials with predictive maintenance within pharmaceutical manufacturing critical component of the clinical trial supply chain. By predicting equipment failures and scheduling timely maintenance, AI technologies minimize downtime and ensure consistent production quality. This application of AI is particularly crucial given the stringent regulatory requirements and high standards of pharmaceutical production. The trend reflects a broader shift towards Industry 4.0 within pharma, where smart factories leverage AI to optimize operations. As generative AI becomes more sophisticated, its role in predictive maintenance is expected to expand, further enhancing operational efficiencies and compliance adherence.
These trends underscore a pivotal year for Generative AI in the Clinical Trials Market, where technological advancements are not only enhancing existing processes but also paving the way for novel approaches in pharmaceutical development. As stakeholders continue to recognize the strategic value of generative AI, its integration across the drug development lifecycle is set to deepen, promising significant impacts on the speed, cost, and efficacy of clinical trials.
Regional Analysis
North America Dominates with 38% Market Share in Generative AI In Clinical Trials Market.
North America, currently the most dominant region, holds approximately 38% of the market share. This dominance is largely attributed to robust technological infrastructure, significant investments in AI and healthcare research, and a regulatory environment conducive to AI adoption in clinical settings. The United States spearheads this region's advancements, with numerous biotechnology and pharmaceutical companies integrating AI to enhance the efficiency and accuracy of clinical trials.
Europe follows, with a strong focus on data protection and patient privacy, underpinned by GDPR compliance. The region is seeing growing adoption of AI in clinical trials, driven by collaborations between AI tech firms and European pharma companies. This growth is further supported by funding from both public and private sectors aimed at advancing healthcare innovation.
Asia Pacific is identified as the fastest-growing region in this market. Countries like China, Japan, and South Korea are making significant strides, driven by government initiatives to support AI integration in healthcare and a rising number of startups focusing on AI-driven clinical trial solutions. This region benefits from a large patient population, which provides a vast data pool for AI applications.
Middle East & Africa and Latin America are emerging players in this field. These regions are beginning to embrace digital transformation in healthcare, with an increasing number of partnerships and investments focusing on developing AI capabilities for clinical research. However, growth in these areas is tempered by challenges such as limited healthcare infrastructure and regulatory frameworks.
Key Regions
North America
- The US
- Canada
- Rest of North America
Europe
- Germany
- France
- The UK
- Spain
- Netherlands
- Russia
- Italy
- Rest of Europe
Asia-Pacific
- China
- Japan
- Singapore
- Thailand
- South Korea
- Vietnam
- India
- New Zealand
- Rest of Asia Pacific
Latin America
- Mexico
- Brazil
- Rest of Latin America
Middle East & Africa
- Saudi Arabia
- South Africa
- UAE
- Rest of the Middle East & Africa
The global Generative AI in Clinical Trials market is expected to witness substantial growth, with key players like IBM Watson, Microsoft Corporation, and Google LLC leading the charge. IBM Watson’s robust AI capabilities, particularly in natural language processing and machine learning, position it as a frontrunner in driving innovation within clinical trials. Microsoft Corporation's Azure platform offers a comprehensive suite of AI tools and services, empowering researchers to leverage advanced analytics for improved trial efficiency and data insights. Meanwhile, Google LLC’s expertise in AI and healthcare data analytics promises to revolutionize clinical trial processes through enhanced patient recruitment, personalized treatment plans, and predictive analytics.
Tencent Holdings Ltd., Neuralink Corporation, and Johnson & Johnson also play significant roles in shaping the landscape of generative AI in clinical trials. Tencent’s vast resources and expertise in AI technologies offer valuable contributions to accelerating the adoption of AI-driven solutions in healthcare. Neuralink Corporation’s focus on developing brain-machine interface technologies holds immense potential for advancing patient monitoring and data collection during clinical trials.
Johnson & Johnson’s commitment to innovation in healthcare, coupled with its strategic investments in AI, positions it as a key player poised to drive advancements in clinical trial methodologies and patient outcomes. Other key players in the market are anticipated to contribute to the ecosystem through niche expertise and collaborative efforts aimed at harnessing the full potential of generative AI in clinical trials.
Market Key Players
- IBM Watson
- Microsoft Corporation
- Google LLC
- Tencent Holdings Ltd.
- Neuralink Corporation
- Johnson & Johnson
- Other Key Players
Recent Developments
- In April 2024, FN Media Group LLC - The global AI in healthcare market is projected to grow at a CAGR of 36.4% from 2024 to 2030, driven by the increasing adoption of AI and machine learning technologies for early disease diagnosis and efficient healthcare management.
- In February 2024, ServiceNow and NVIDIA announced an expansion of their partnership with the launch of telco-specific generative AI solutions aimed at enhancing service experiences through the Now Platform.
- In January 2024, EY India revealed that 48% of healthcare and pharma companies plan to integrate their first GenAI solution within the next year, underscoring a significant shift towards AI adoption despite existing challenges in preparedness and skills.
Report Scope
Report Features Description Market Value (2023) USD 140 Million Forecast Revenue (2033) USD 275.8 Million CAGR (2024-2032) 23.8% Base Year for Estimation 2023 Historic Period 2016-2023 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Application (Data generation, Clinical trial design, Outcome prediction, Adverse event detection, Data imputation and Denoising, and Other Applications); By Technology (Variational Autoencoders (VAEs), Generative Adversarial Networks (GANs), Deep Convolutional Networks (DCNs), Transfer Learning, and Other Technologies); By End-Use (Researchers and Scientists, Healthcare Professionals, Clinical Trial Sponsors and CROs, Data Analysts and Biostatisticians, and Other End Uses) Regional Analysis North America - The US, Canada, Rest of North America, Europe - Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe, Asia-Pacific - China, Japan, South Korea, India, New Zealand, Singapore, Thailand, Vietnam, Rest of Asia Pacific, Latin America - Brazil, Mexico, Rest of Latin America, Middle East & Africa - South Africa, Saudi Arabia, UAE, Rest of Middle East & Africa Competitive Landscape IBM Watson, Microsoft Corporation, Google LLC, Tencent Holdings Ltd., Neuralink Corporation, Johnson & Johnson, and Other Key Players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- IBM Watson
- Microsoft Corporation
- Google LLC
- Tencent Holdings Ltd.
- Neuralink Corporation
- Johnson & Johnson
- Other Key Players




