Dengue Vaccine Market By Type(CYD-TDV, Tak-003, Other), By Category(Pediatric, Adult), By Route of Administration(Subcutaneous, Intravenous), By End User(Hospitals, Clinics, Government Institutes, Non-Governmental Organizations (NGOs), Others), By Region And Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, And Forecast 2024-2033
-
38257
-
March 2024
-
179
-
-
This report was compiled by Correspondence Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Report Overview
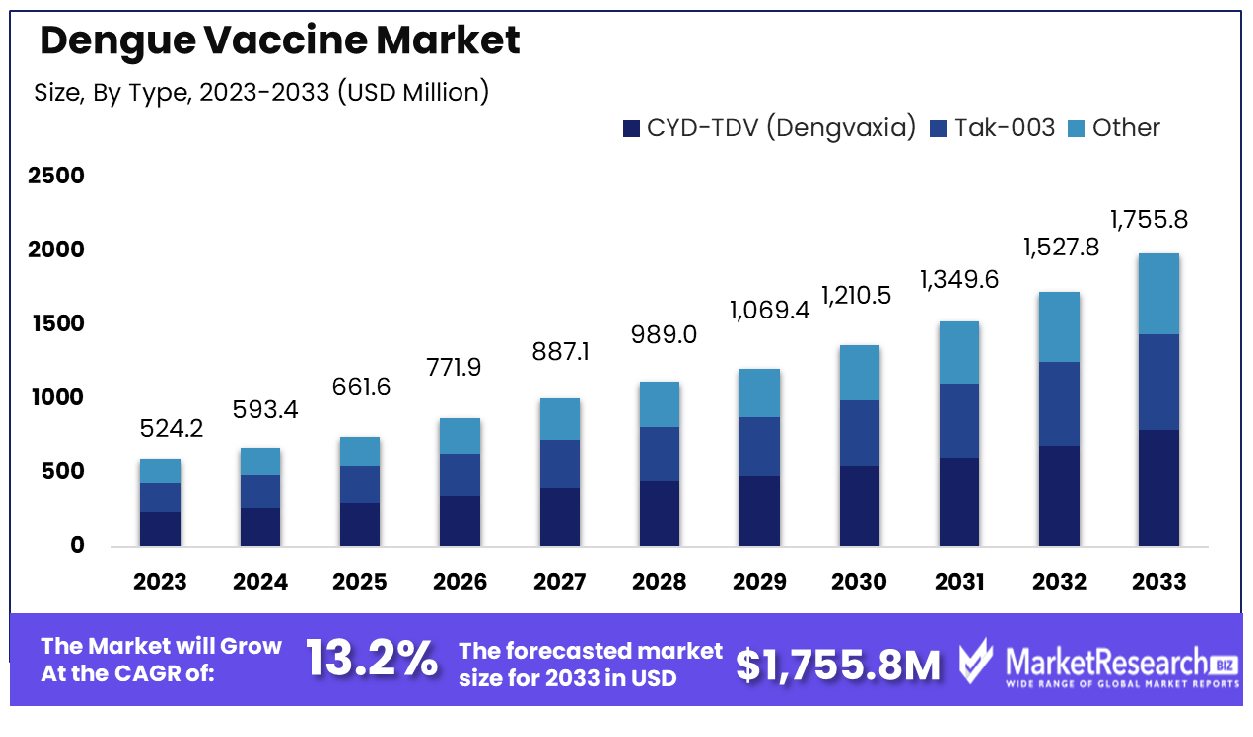
The global Dengue Vaccine Market was valued at USD 524.2 million in 2023, It is expected to reach USD 1,755.8 million by 2033, with a CAGR of 13.2% during the forecast period from 2024 to 2033.
The surge in demand for medical vaccines and the rise in dengue cases are some of the main key driving factors for the dengue vaccine market. The dengue vaccine is defined as the medical intercession created to offer immunity against the dengue virus which is transmitted to humans through infected mosquito bites mainly Aedes Aegypti.
Many vaccines individuals have been created to fight dengue fever, a viral infection that causes flu-like symptoms. Such vaccines, Dengvaxia have been approved in some of the regions. Dengue vaccines specifically target all four serotypes of the virus to avoid severe illness and complications, as infections with one serotype do not confer immunity against the others.
These vaccinations focus on decreasing the risk of dengue infections, severe disease, and associated hospitalizations. Current research and development efforts are aimed at enhancing vaccine efficiency, accessibility, and safety that consider the world impact of dengue as a main public health issue in tropical and subtropical countries. Furthermore, challenges are still continuous, and careful attention is given to factors like serotype interactions and capable vaccine-related concerns to ensure the efficacy, effectiveness, and safety of dengue vaccines.
MSN in February 2024, highlights that Takeda, a Japanese pharma leader has entered into a tactical collaboration with Biological E limited, a Hyderabad-based biopharma player that will produce up to 50 million doses of Takeda’s dengue vaccine QDENGA per annum. Moreover, this partnership fastens its potential to offer 100 million doses of the dengue vaccines by 2030. Presently, QDENGA is available for children and adults in the private market in regions in Europe as well as Indonesia and Thailand and in private and some public programs in private and certain public programs in Argentina and Brazil. TAK-003 is not yet approved for use in India.
Dengue vaccines hold great significance in public health as they play an important role in avoiding and regulating the spread of dengue fever, and mosquito-borne viral infections. With the surge in world dengue cases, vaccines offer a proactive way to decrease the incidence of severe diseases, mortality related to the virus, and hospitalizations. Their utilization can highly contribute to mitigating the socioeconomic burden of dengue outbreaks, shielding the vulnerable populations, and supporting whole efforts to fight dengue on a broader scale. The demand for dengue vaccines will increase due to its requirement in various hospitals and healthcare hubs which will help in market expansion in the coming years.
Key Takeaways
- Market Growth: The Global Dengue Vaccine Market was valued at USD 524.2 million in 2023, It is expected to reach USD 1,755.8 million by 2033, with a CAGR of 13.2% during the forecast period from 2024 to 2033.
- By Type: CYD-TDV (Dengvaxia) dominates its segment in terms of type in current market dynamics.
- By Category: Pediatric segment reigns supreme in terms of category within the market landscape.
- By Route of Administration: Subcutaneous route of administration emerges as the dominant segment in current trends.
- By End User: Hospitals emerge as the dominant end-user segment within the market's landscape.
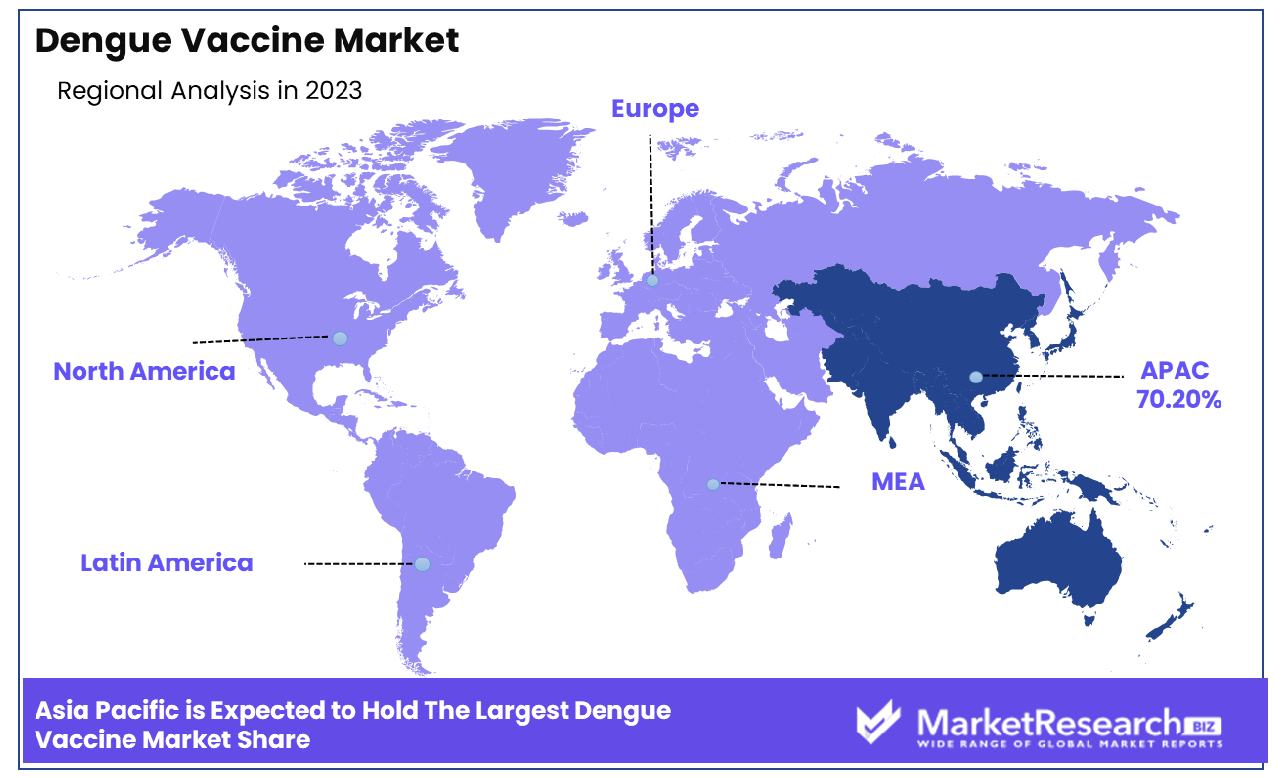
- Regional Dominance: Asia Pacific Dominates with 70.20% Market Share in the Dengue Vaccine Market
- Growth Opportunity: Heightened research initiatives and expanded vaccination campaigns drive growth in the Dengue Vaccine Market, addressing unmet medical needs and extending preventive measures to new geographic regions vulnerable to dengue outbreaks.
Driving factors
Development of Broad Age Range Vaccines Drives Dengue Vaccine Market Growth
Focusing on creating dengue vaccines suitable for individuals aged four to 60 is significantly fuelling market growth. This broad age range covers most of those at risk from dengue, making this product development effort critical in controlling its spread. By creating vaccines that are effective and safe across an extended age spectrum, manufacturers are broadening the potential consumer base to address an important public health need.
This development is of particular significance in dengue-endemic regions, where this disease poses a threat to an overwhelming portion of society regardless of age. Over time, its long-term impacts will include strengthened herd immunity against dengue as well as potential reductions in disease prevalence - contributing positively to both public health and economic stability in affected regions.
Comprehensive Protection: Multi-Strain Vaccines Gain Market Acceptance
The availability of vaccines offering protection against multiple strains of the dengue virus, coupled with demonstrated efficacy and safety, is enhancing market acceptance and growth analysis. Dengue, caused by four different virus serotypes, requires a vaccine that can provide broad protection. Vaccines that effectively address multiple strains not only offer comprehensive protection but also increase consumer confidence due to their proven efficacy and safety profile.
This factor is particularly pivotal in gaining regulatory approvals and public trust, essential elements for widespread vaccine adoption. In the long run, the development and acceptance of multi-strain vaccines are expected to solidify the market's growth, paving the way for increased vaccine coverage and potentially impacting global dengue incidence rates.
Pharma Giants Fuel Vaccine Research and Development
Pharmaceutical companies' active participation in developing dengue vaccines is driving innovation and market expansion. By investing considerable resources and expertise into research and development efforts to produce more effective dengue vaccines, these firms are helping overcome scientific and logistical challenges associated with vaccine development, from initial research through clinical trials and mass production.
The involvement of established pharmaceutical major players not only accelerates vaccine development but also ensures adherence to global safety and efficacy standards. This factor is anticipated to lead to a more competitive market with multiple vaccine options, ultimately benefiting public health initiatives against dengue.
Growing Demand for Innovative Vaccines Boosts Market Growth
Dengue vaccine sales have seen rapid expansion as a result of the rising global dengue burden, and as its global burden continues to expand so too does its need for innovative vaccine solutions to control outbreaks and reduce dengue symptoms. This rising demand has spurred investments into vaccine technology such as DNA vaccines, vector-based vaccines, and advanced adjuvants that enhance immune responses - driving growth across this sector of healthcare spending.
This pursuit of innovation is not only addressing current healthcare challenges but is also preparing the market for future needs, considering potential changes in virus behavior and epidemiology. The long-term effect of this demand for innovation is likely to result in more effective and adaptable dengue vaccines, ensuring preparedness for future dengue challenges.
Restraining Factors
Limited Efficacy of Current Vaccines Restrains Dengue Vaccine Market Growth
Dengue vaccine market growth is limited by the limited efficacy of current vaccines. Current dengue vaccines exhibit variable efficacy rates across populations, particularly age groups and those previously exposed or not previously exposed to the dengue virus. This variability leads to healthcare providers and patients being reluctant to use it resulting in a lower adoption rate; further complicating its growth potential.
Stringent Regulatory Requirements for Vaccine Approval Restrains Market Growth
Stringent regulatory requirements for vaccine approval significantly limit the growth of the dengue market. Developing a vaccine that meets all safety and efficacy standards is a complex and time-consuming process, often taking several years to complete. These regulatory hurdles can delay the entry of new vaccines into the market and can also increase development costs. The intense scrutiny and the need for extensive clinical trials to establish safety and efficacy profiles mean that only a few candidates reach the market, limiting the availability and diversity of options.
By Type Analysis
CYD-TDV, commonly known as Dengvaxia, dominates the segment concerning type within the pharmaceutical market landscape.
In 2023, CYD-TDV (Dengvaxia) held a dominant market position in the By Type segment of the Dengue Vaccine Market. The market segmentation primarily consisted of CYD-TDV (Dengvaxia), Tak-003, and Other variants. CYD-TDV (Dengvaxia) commanded a significant share owing to its early market entry, extensive clinical research, and established safety profile. With its efficacy against dengue fever demonstrated across various age groups, CYD-TDV emerged as the preferred choice for healthcare providers and governments in dengue-endemic regions.
Tak-003, though promising, faced regulatory hurdles and slower adoption rates, limiting its market penetration. Other variants encompassed a range of emerging candidates undergoing clinical trials or awaiting regulatory approvals. Despite the competition, CYD-TDV maintained its stronghold, benefiting from brand recognition, distribution networks, and strategic partnerships.
The growth of the market can be attributed to increasing awareness campaigns, government initiatives for immunization, and rising incidences of dengue infections globally. Looking ahead, advancements in vaccine technology and expanding access to preventive healthcare are poised to drive further growth in the Dengue Vaccine Market.
By Category Analysis
In the pediatric category, products hold sway, capturing a dominant segment share in the pharmaceutical industry.
In 2023, Pediatrics held a dominant market position in the By Category segment of the Dengue Vaccine Market. The significant prevalence of dengue fever among children, coupled with extensive vaccination campaigns in pediatric populations across various regions, propelled the Pediatric segment to the forefront. Pediatric vaccines are tailored to address the unique immunological needs of children, ensuring optimal efficacy and safety profiles.
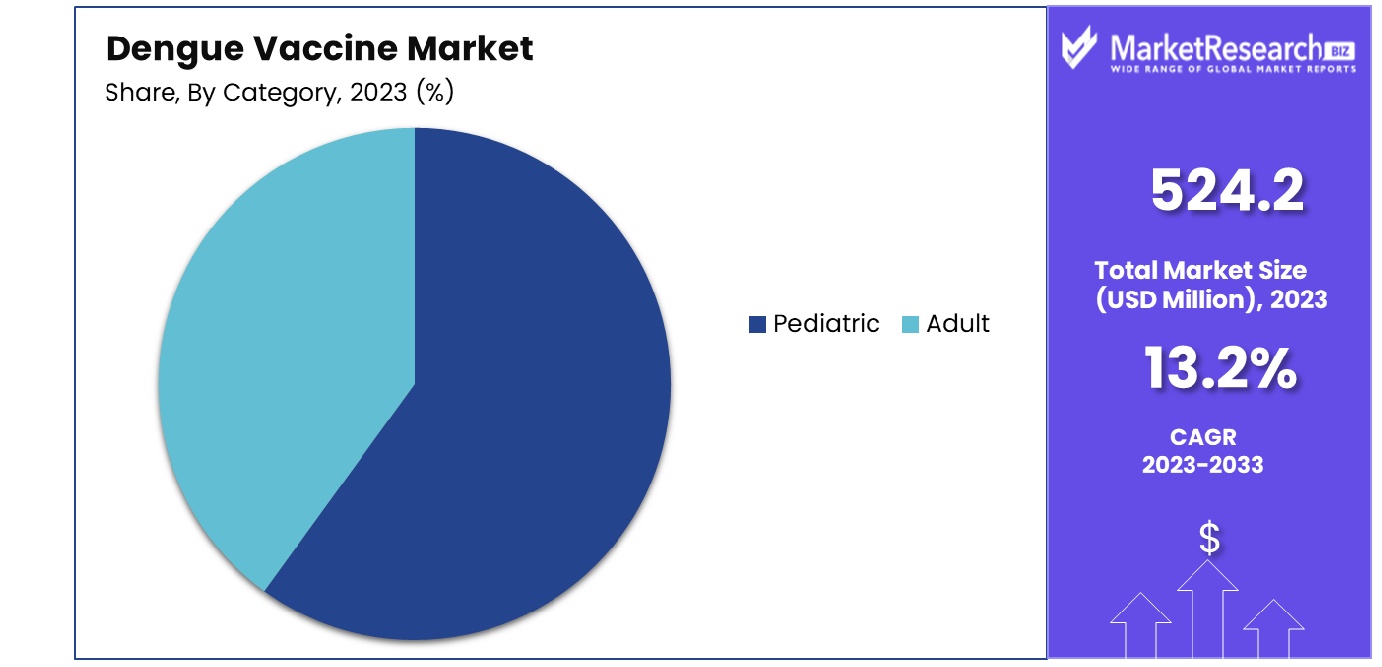
The Pediatric segment's dominance underscores the prioritization of pediatric vaccination programs by healthcare authorities and organizations worldwide. This emphasis on early immunization not only aims to curb the burden of dengue fever among children but also contributes to long-term disease prevention and public health initiatives.
Furthermore, advancements in vaccine development technologies have facilitated the production of pediatric-specific formulations with enhanced immunogenicity and reduced adverse effects, further bolstering the segment's market presence. With a growing focus on preventive healthcare and routine immunization schedules for children, the Pediatric segment is poised for sustained growth and innovation in the Dengue Vaccine Market.
As healthcare systems continue to prioritize pediatric vaccination strategies and expand access to dengue vaccines, the Pediatric segment is anticipated to maintain its dominant position, driving market expansion and fostering positive public health outcomes.
By Route of Administration Analysis
Subcutaneous administration stands out as the dominant segment in terms of the route of administration in pharmaceuticals.
In 2023, Subcutaneous held a dominant market position in the By Route of Administration segment of the Dengue Vaccine Market. The subcutaneous route of administration emerged as a preferred choice due to its efficacy and ease of administration, driving substantial market growth. With its advantages of reduced injection-related discomfort and improved patient compliance, the subcutaneous route garnered significant traction among healthcare providers and patients alike.
Additionally, advancements in drug delivery technologies further propelled the adoption of subcutaneous administration for dengue vaccines. These technologies facilitated precise dosage delivery and enhanced vaccine stability, addressing key challenges associated with conventional administration methods.
In contrast, the intravenous route, while offering certain benefits such as rapid onset of action, faced limitations concerning practicality and accessibility in routine vaccination programs. Consequently, the market witnessed a pronounced preference for subcutaneous administration, underpinned by its favorable balance of efficacy, safety, and patient convenience.
Looking ahead, the dominance of the subcutaneous route is poised to persist, fueled by ongoing innovations in vaccine delivery systems and increasing emphasis on improving immunization coverage globally. As stakeholders continue to prioritize efficient and patient-friendly vaccination strategies, the subcutaneous route is expected to maintain its stronghold in the Dengue Vaccine Market, driving sustained market expansion and benefiting both healthcare providers and patients.
By End User Analysis
Hospitals emerged as the dominant segment among end users within the pharmaceutical market, particularly in medical product distribution.
In 2023, Hospitals held a dominant market position in the By End User segment of the Dengue Vaccine Market. Hospitals emerged as key stakeholders due to their pivotal role in administering vaccines and managing disease outbreaks. Their extensive infrastructure, including specialized departments for infectious diseases and vaccination programs, positioned them as primary distribution points for dengue vaccines.
Additionally, hospitals often serve as referral centers for severe dengue cases, driving the demand for preventive measures such as vaccines. Clinics, while playing a significant role, trailed hospitals in market share due to their comparatively limited capacity for mass vaccination campaigns. Government Institutes also contributed substantially, leveraging public health initiatives and immunization drives to distribute vaccines across diverse demographics.
Non-Governmental Organizations (NGOs) complemented these efforts, particularly in underserved regions, by conducting awareness campaigns and providing vaccines to vulnerable populations. Other entities, such as pharmacies and corporate healthcare facilities, played niche roles in vaccine distribution. Overall, Hospitals emerged as the cornerstone of the Dengue Vaccine Market, facilitating widespread access and uptake of preventive measures against dengue fever.
Key Market Segments
By Type
- CYD-TDV
- Tak-003
- Other
By Category
- Pediatric
- Adult
By Route of Administration
- Subcutaneous
- Intravenous
By End User
- Hospitals
- Clinics
- Government Institutes
- Non-Governmental Organizations (NGOs)
- Others
Growth Opportunity
Enhanced Research Initiatives Drive Dengue Vaccine Market Expansion
The Dengue Vaccine Market witnesses significant growth opportunities driven by heightened research initiatives aiming to develop efficacious and accessible vaccines against the dengue virus. Collaborations between research institutions, pharmaceutical companies, and governmental organizations aim to address the unmet medical need posed by dengue fever, particularly in endemic regions.
Advanced vaccine candidates undergoing clinical trials, coupled with substantial investments in vaccine development programs, pave the way for novel preventive measures against dengue natural infection. The focus on developing affordable and scalable vaccine solutions, alongside expanded immunization programs in dengue-endemic regions, underscores the market's potential for growth and impact in mitigating the global burden of dengue fever.
Expanding Geographic Reach Amplifies Dengue Vaccine Market Scope
The Dengue Vaccine Market witnesses significant growth opportunities driven by heightened research initiatives aiming to develop efficacious and accessible vaccines against the dengue virus. Collaborations between research institutions, pharmaceutical companies, and governmental organizations aim to address the unmet medical need posed by dengue fever, particularly in endemic regions.
Advanced vaccine candidates undergoing clinical trials, coupled with substantial investments in vaccine development programs, pave the way for novel preventive measures against dengue infection. The focus on developing affordable and scalable vaccine solutions, alongside expanded immunization programs in dengue-endemic regions, underscores the market's potential for positive growth and impact in mitigating the global burden of dengue fever.
The year 2019 had the highest historical dengue caseload before 2023, with over 3.18 million cases reported globally. Additionally, in January 2024, over half a million dengue cases and over 100 dengue-related deaths were reported globally. These statistics underscore the urgent need for effective preventive measures against dengue fever, further emphasizing the significance of ongoing research and development efforts in the Dengue Vaccine Market.
Latest Trends
Advancements in Dengue Vaccine Development Revolutionize Market Landscape
Recent trends in the Dengue Vaccine Market reveal a surge in research and development activities aimed at enhancing vaccine efficacy and accessibility. Advancements in vaccine technology, including novel formulations and delivery methods such as DNA vaccines and nanoparticle-based platforms, offer promising prospects for improved immunization against dengue virus strains. Additionally, the integration of innovative adjuvants and immune-modulators enhances vaccine potency and duration of protection, fostering increased adoption and effectiveness in vulnerable populations.
Furthermore, the market witnesses a growing emphasis on multi-strain and tetravalent vaccine formulations, addressing the challenges posed by the complex nature of dengue virus serotypes. These next-generation vaccines aim to provide broad-spectrum protection against all four dengue virus strains, offering a comprehensive solution to combat the global burden of dengue fever. Collaborative efforts between vaccine manufacturers, research institutions, and public health organizations accelerate vaccine development timelines and facilitate regulatory approvals, expediting market entry and commercialization of novel dengue vaccines.
Digital Solutions Transform Dengue Vaccine Distribution and Surveillance
Recent trends in the Dengue Vaccine Market underscore the pivotal role of digital solutions in optimizing vaccine distribution and surveillance efforts. The integration of digital platforms and data analytics tools enables real-time monitoring of vaccine supply chains, enhancing inventory management, and ensuring timely delivery to endemic regions. Additionally, innovative mobile applications and web-based platforms facilitate vaccine tracking, adverse event reporting, and population-level surveillance, empowering healthcare authorities to implement targeted immunization campaigns and monitor vaccine effectiveness.
Furthermore, the adoption of blockchain technology ensures transparency and traceability in vaccine distribution processes, mitigating the risk of counterfeit vaccines and supply chain disruptions. Leveraging artificial intelligence and machine learning algorithms enables predictive modeling of dengue outbreaks, enabling proactive public health interventions and resource allocation. Collaborative initiatives between governments, non-profit organizations, and technology providers drive the implementation of digital solutions across the dengue vaccine ecosystem, revolutionizing disease prevention strategies and ultimately reducing the global burden of dengue fever.
Regional Analysis
Asia Pacific Dominates with 70.20% Market Share in the Dengue Vaccine Market
Asia Pacific region accounts for 70.20% of the global dengue vaccine market, driven by high prevalence rates in countries like India, Thailand, and the Philippines. This region's significant share can be attributed to factors like its dense population density, a tropical climate conducive to mosquito breeding, mosquito-borne disease, and historically high infection rates which create immense demand for effective vaccination solutions.
Market dynamics are affected by government initiatives to control and prevent dengue outbreaks, including national immunization programs and public awareness campaigns. International health organizations playing an active role in funding vaccination programs also play a crucial role.
Forecast projections predict that Asia Pacific will remain the leader in the dengue vaccine market for some time to come. Ongoing efforts to manage outbreaks, as well as new and more effective vaccines being developed, are likely to drive this market's expansion. Furthermore, rising healthcare expenditure and an emphasis on preventive medicine in emerging economies should all help maintain this region's market dominance over time.
Latin America:
Latin America is another significant market for dengue vaccines due to the prevalence of dengue fever in endemic countries like Brazil and Mexico. Warm climate conditions combined with socioeconomic influences play a part in spreading this infectious disease - creating a strong demand for effective vaccination measures in this part of the world.
Latin American markets are anticipated to experience strong market analysis due to public health initiatives and awareness about dengue prevention. Government efforts integrating dengue vaccines into national immunization programs will play a vital role in this anticipated expansion.
North America:
North America's dengue vaccine market is growing quickly, driven by increasing travel to dengue-endemic regions and potential spread to parts of the United States. While its current size is relatively modest compared to the Asia Pacific and Latin America markets, increasing global mobility could increase outbreaks across this region.
North America's market for dengue vaccines and preventive healthcare measures for travelers is projected to expand steadily. Research and development efforts continue, while more travelers focus on preventative healthcare measures as a means of staying healthy while traveling abroad.
Key Regions and Countries
North America
- The US
- Canada
- Rest of North America
Europe
- Germany
- France
- The UK
- Spain
- Netherlands
- Russia
- Italy
- Rest of Europe
Asia-Pacific
- China
- Japan
- Singapore
- Thailand
- South Korea
- Vietnam
- India
- New Zealand
- Rest of Asia Pacific
Latin America
- Mexico
- Brazil
- Rest of Latin America
Middle East & Africa
- Saudi Arabia
- South Africa
- UAE
- Rest of Middle East & Africa
Key Players Analysis
The dengue vaccine market is shaped by the strategic initiatives of several pharmaceutical giants and specialized vaccine developers. Sanofi Pasteur, as the pioneer with its first-ever approved dengue vaccine, holds a significant position, influencing market dynamics and setting standards for safety and efficacy.
Takeda Pharmaceutical Company and the Butantan Institute are notable for their advanced clinical trials and innovative approaches to developing more effective dengue vaccines, signaling a strong commitment to addressing this global health challenge.
Medigen Vaccine Biologics Corporation and GlaxoSmithKline Plc have contributed to the market with their research and development expertise, focusing on novel vaccine technologies to enhance immunogenicity and safety profiles.
Panacea Biotec Limited and Serum Institute of India are key players in expanding the market's reach, especially in endemic regions, by focusing on accessible and affordable vaccine diseases. Merck And Co. Inc., with its extensive development in vaccines, is a potential influencer in shaping future vaccine strategies.
Sun Pharmaceutical Industries Ltd and Mylan N.V., primarily known for generics, strengthen the market with their distribution networks and potential to make dengue vaccines more accessible. Teva Pharmaceutical Industries Ltd. and Novartis AG, though more diversified, contribute to the overall research and potential future offerings in the dengue vaccine landscape.
Market Key Players
- Sanofi Pasteur Limited
- Takeda Pharmaceutical Company
- Butantan Institute
- Medigen Vaccine Biologics Corporation
- GlaxoSmithKline Plc
- Panacea Biotec Limited
- Merck And Co. Inc.
- Sun Pharm
- GeneOne Life Science Inc
- Nunn’s Home Medical Equipment Company Ltd.
- Serum Institute Of ...
- Mylan N.V.
- Teva Pharmaceutical Industries Ltd.
- Novartis AG
- GSK plc
- F. Hoffmann-La Roche Ltd.
- Institute Of India Pvt. Ltd.
Recent Development
- In February 2024, Sandoz settles US antitrust litigation, agreeing to a $265 million payment without admission of wrongdoing. The settlement covers direct purchaser claims from 2009-2019, pending court approval. Sandoz Group AG focuses on generic pharmaceuticals.
- In February 2024, Takeda partnered with Biological E to produce 50 million dengue vaccines. Miltenyi Biotec establishes the Hyderabad Center for Cell and Gene Therapy services. BioAsia 2024 showcases pharmaceutical industry advancements.
- In February 2024, Dengue continues to pose a global threat, with outbreaks in Africa, the Americas, and Southeast Asia. Vaccine development efforts face challenges, with Takeda and Sanofi encountering efficacy issues.
Report Scope
Report Features Description Market Value (2023) USD 524.2 Million Forecast Revenue (2033) USD 1,755.8 Million CAGR (2024-2032) 13.2% Base Year for Estimation 2023 Historic Period 2016-2023 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Type(CYD-TDV, Tak-003, Other), By Category(Pediatric, Adult), By Route of Administration(Subcutaneous, Intravenous), By End User(Hospitals, Clinics, Government Institutes, Non-Governmental Organizations (NGOs), Others) Regional Analysis North America - The US, Canada, Rest of North America, Europe - Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe, Asia-Pacific - China, Japan, South Korea, India, New Zealand, Singapore, Thailand, Vietnam, Rest of Asia Pacific, Latin America - Brazil, Mexico, Rest of Latin America, Middle East & Africa - South Africa, Saudi Arabia, UAE, Rest of Middle East & Africa Competitive Landscape Sanofi Pasteur Limited, Takeda Pharmaceutical Company, Butantan Institute, Medigen Vaccine Biologics Corporation, GlaxoSmithKline Plc, Panacea Biotec Limited, Merck And Co. Inc., Sun Pharm, GeneOne Life Science Inc, Nunn’s Home Medical Equipment Company Ltd., Serum Institute Of, Mylan N.V., Teva Pharmaceutical Industries Ltd., Novartis AG, GSK plc, F. Hoffmann-La Roche Ltd., Institute Of India Pvt. Ltd. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Sanofi Pasteur Limited
- Takeda Pharmaceutical Company
- Butantan Institute
- Medigen Vaccine Biologics Corporation
- GlaxoSmithKline Plc
- Panacea Biotec Limited
- Merck And Co. Inc.
- Sun Pharm
- GeneOne Life Science Inc
- Nunn’s Home Medical Equipment Company Ltd.
- Serum Institute Of ...
- Mylan N.V.
- Teva Pharmaceutical Industries Ltd.
- Novartis AG
- GSK plc
- F. Hoffmann-La Roche Ltd.
- Institute Of India Pvt. Ltd.




