
Tuberculosis Vaccine Treatment Market By Type (Immunotherapeutic Vaccines, Booster Vaccines, Others), By Application (Hospitals, Clinics, Research Institutes, Others), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
-
45854
-
April 2024
-
136
-
-
This report was compiled by Trishita Deb Trishita Deb is an experienced market research and consulting professional with over 7 years of expertise across healthcare, consumer goods, and materials, contributing to over 400 healthcare-related reports. Correspondence Team Lead- Healthcare Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Quick Navigation
Report Overview
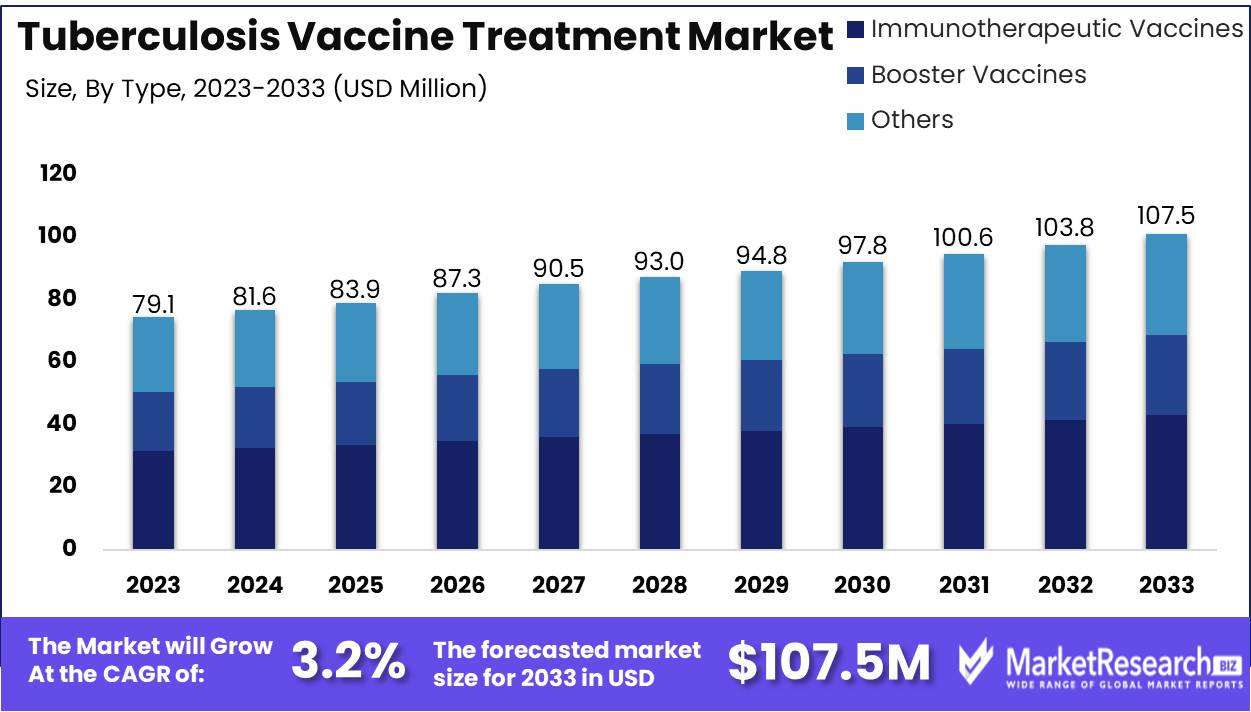
The Global Tuberculosis Vaccine Treatment Market was valued at USD 79.1 Mn in 2023. It is expected to reach USD 107.5 Mn by 2033, with a CAGR of 3.2% during the forecast period from 2024 to 2033.
The Tuberculosis Vaccine Treatment Market encompasses the dynamic landscape of products, therapies, and innovations aimed at combatting tuberculosis (TB), a global health challenge. This market segment focuses on the development, manufacturing, distribution, and adoption of vaccines and treatments designed to prevent and manage TB infections. With a pressing need for effective solutions against this persistent disease, stakeholders within this market engage in rigorous research, clinical trials, and regulatory processes to bring promising therapies to fruition. As a pivotal component in the fight against TB, this market offers opportunities for strategic partnerships, investment, and advancements that can drive meaningful impact on public health worldwide.
The Tuberculosis Vaccine Treatment Market presents a nuanced landscape influenced by a confluence of factors. Tuberculosis, afflicting over 10 million individuals globally each year, underscores the critical need for effective preventive and therapeutic measures. The efficacy range of the Bacille Calmette-Guérin (BCG) vaccine, spanning from 0-80% in preventing severe TB in children, emphasizes the market's ongoing challenge in achieving consistent efficacy across populations. Despite BCG's widespread use, the variability in its effectiveness underscores the imperative for continued research and development efforts within this market segment.
The market's dynamism is further fueled by evolving disease patterns, emerging strains of drug-resistant TB, and shifting healthcare priorities. Stakeholders in this market, ranging from pharmaceutical companies to public health organizations, are propelled to innovate and collaborate to address these complexities effectively.
To navigate this landscape successfully, stakeholders must adopt a multifaceted approach, encompassing robust research and development pipelines, strategic partnerships, and targeted interventions. Investments in novel vaccine technologies, therapeutic modalities, and precision medicine approaches hold promise for driving advancements in TB prevention and treatment.
Key Takeaways
- Market Value: The Global Tuberculosis Vaccine Treatment Market was valued at USD 79.1 Mn in 2023. It is expected to reach USD 107.5 Mn by 2033, with a CAGR of 3.2% during the forecast period from 2024 to 2033.
- By Type: Immunotherapeutic Vaccines assert dominance, holding a commanding share of 40%, underscoring their pivotal role in tuberculosis treatment.
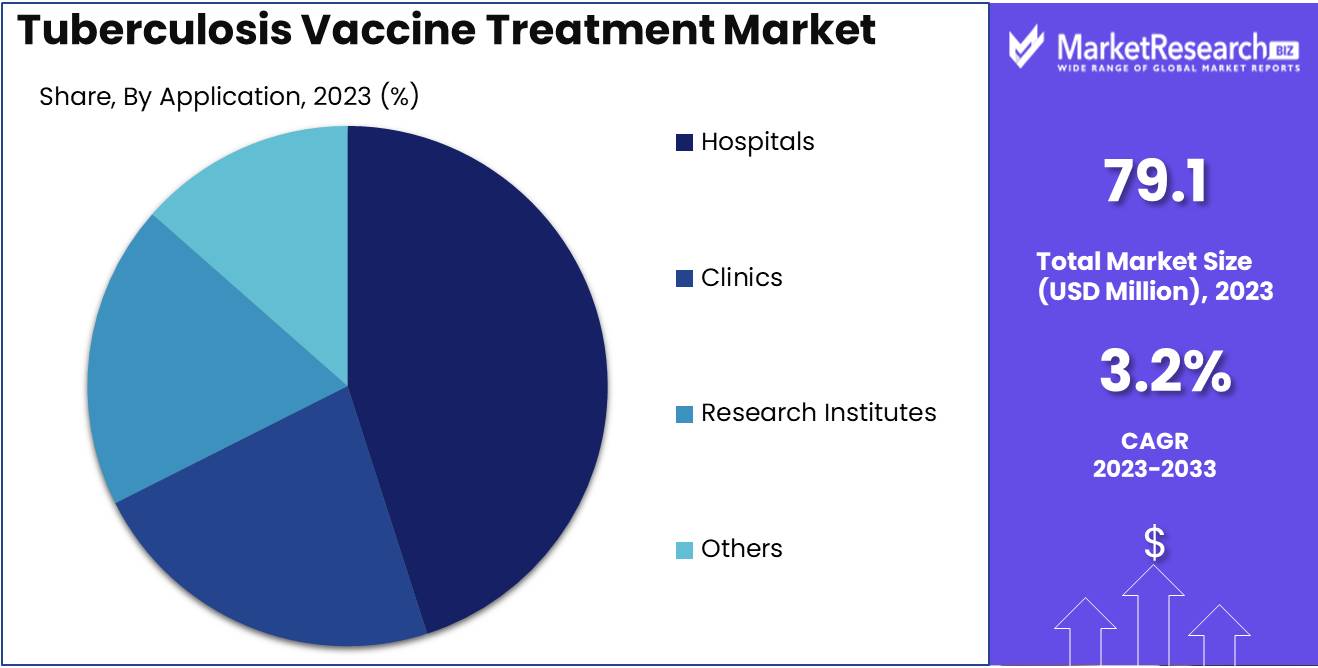
- By Application: Hospitals emerge as the primary stronghold, commanding a significant share of 50%, highlighting their crucial role in tuberculosis management and patient care.
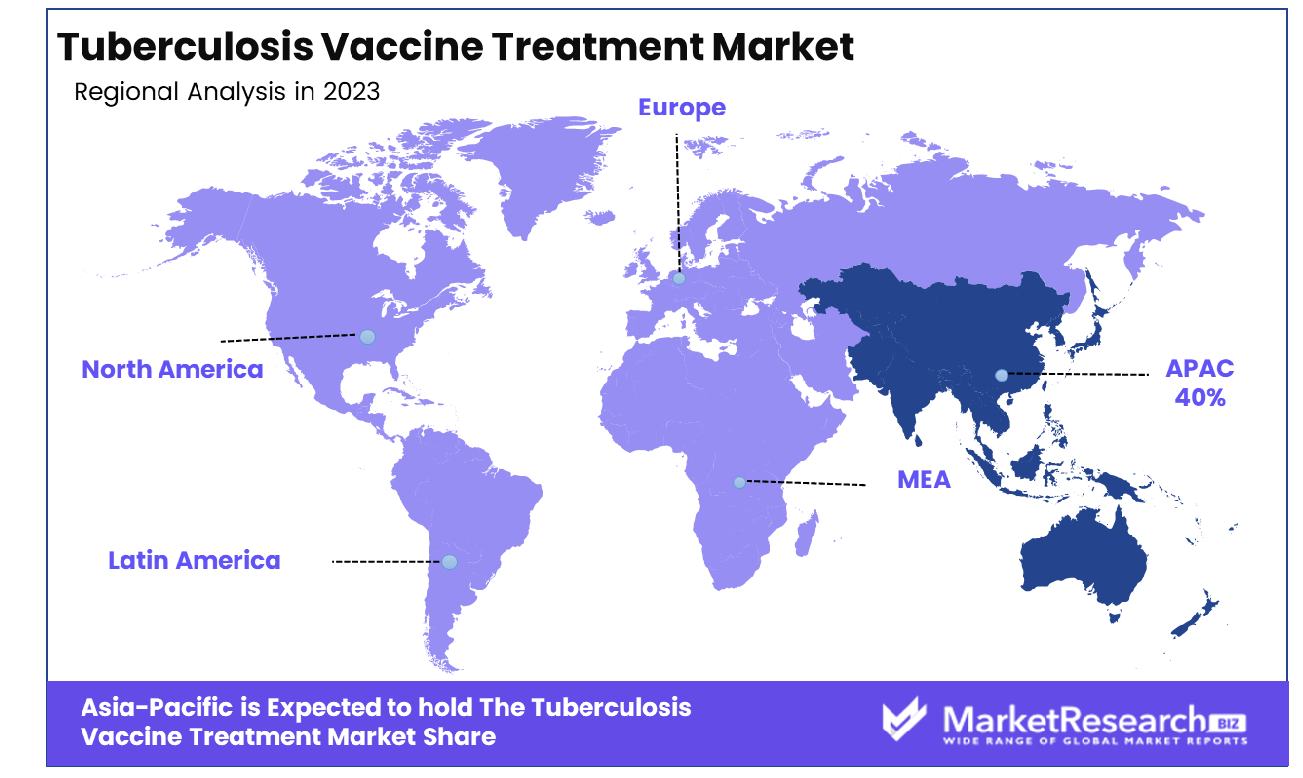
- Regional Dominance: Asia-Pacific region, commanding a significant share of 40%, highlighting the region's focus on innovative treatment modalities for tuberculosis.
- Growth Opportunity: This market presents ample opportunities for growth through continuous advancements in vaccine research and development, aiming to enhance efficacy and accessibility worldwide.
Driving factors
Rising Demand for TB Vaccines
The escalating demand for tuberculosis (TB) vaccines is a pivotal driver propelling the growth of the TB vaccine treatment market. Despite concerted efforts to control TB, it remains a significant global health burden, particularly in regions with limited access to healthcare resources. With an estimated 10 million new cases annually, there is a pressing need for effective preventive measures, including vaccines. As awareness about TB and its prevention spreads, demand for vaccines is expected to rise further.
The global TB vaccine market is witnessing a surge in demand due to various factors such as population growth, urbanization, and increasing travel, all of which contribute to the spread of TB. Additionally, the emergence of drug-resistant strains of TB underscores the importance of vaccination as a preventive strategy. Pharmaceutical companies are responding to this demand by intensifying their efforts in TB vaccine research and development, aiming to introduce more efficacious and accessible vaccines to the market.
Advancements in Vaccine Development
Advancements in vaccine development play a pivotal role in shaping the growth trajectory of the TB vaccine treatment market. Over the years, significant progress has been made in understanding the biology of Mycobacterium tuberculosis, the causative agent of TB, as well as the host immune response to the infection. These insights have paved the way for the development of novel vaccine candidates with enhanced efficacy and safety profiles.
In recent years, several promising TB vaccine candidates have entered clinical trials, fueling optimism within the scientific community. These candidates leverage innovative approaches such as recombinant DNA technology, viral vectors, and adjuvants to stimulate robust immune responses against TB. Moreover, advancements in vaccine delivery systems, such as microneedle patches and aerosol formulations, hold the promise of improving vaccine accessibility and compliance, particularly in resource-limited settings.The collaboration between academia, industry, and governmental organizations has been instrumental in driving vaccine development efforts forward.
Government Initiatives and Funding
Government initiatives and funding constitute a cornerstone of the TB vaccine treatment market's growth by providing essential support for research, development, and access initiatives. Recognizing the public health imperative posed by TB, governments worldwide have intensified their efforts to combat the disease, allocating substantial resources to TB control programs and vaccine development initiatives.
Governmental organizations such as the World Health Organization (WHO) and national health agencies play a pivotal role in shaping the TB vaccine landscape through policy formulation, regulatory oversight, and strategic investments. For instance, the WHO's End TB Strategy prioritizes the development and deployment of new TB vaccines as a core pillar of TB control efforts, thereby catalyzing industry interest and investment in TB vaccine research.
Restraining Factors
Challenges in Vaccine Distribution and Administration
One of the foremost challenges in vaccine distribution and administration is the logistical complexity involved in reaching every corner of a region or country. Vaccines often require specific storage conditions, like ultra-low temperatures for mRNA vaccines such as Pfizer-BioNTech and Moderna. This necessitates a robust cold chain infrastructure, which may be lacking in many regions, especially in developing countries. Even in developed nations, the scale of distribution poses significant challenges. Coordinating the transportation of millions of doses, ensuring timely delivery, and maintaining the integrity of the vaccine throughout the process require meticulous planning and execution.
The equitable distribution of vaccines presents a moral and logistical dilemma. There's a risk that wealthier countries or regions might monopolize the available vaccine doses, leaving poorer nations at a disadvantage. This exacerbates global health inequalities and prolongs the pandemic as the virus continues to spread in unprotected populations.
Limited Public Health Infrastructure
Another significant challenge in vaccine distribution and administration is the inadequacy of public health infrastructure in many regions. This includes insufficient healthcare facilities, personnel, and information systems necessary for efficient vaccination campaigns. In rural or remote areas, accessing healthcare services can be particularly challenging, making it harder to reach vulnerable populations. Additionally, misinformation and vaccine hesitancy further strain already fragile healthcare systems, leading to delays and inefficiencies in vaccine rollout efforts.
Addressing the limitations of public health infrastructure requires substantial investment and long-term planning. Strengthening healthcare systems to handle mass vaccination campaigns involves training personnel, upgrading facilities, and implementing robust data management systems to track vaccine distribution and adverse reactions effectively. Furthermore, community engagement and education programs are vital to combat misinformation and build trust in vaccines, ensuring widespread acceptance and uptake across diverse populations.
By Type Analysis
Immunotherapeutic Vaccines led the Tuberculosis Vaccine Treatment Market's By Type segment with over 40% share.
In 2023, Immunotherapeutic Vaccines held a dominant market position in the By Type segment of the Tuberculosis Vaccine Treatment Market, capturing more than a 40% share. Immunotherapeutic Vaccines spearheaded the market with its innovative approach towards combating tuberculosis, leveraging the body's immune system to target and eliminate the disease-causing pathogens effectively. The efficacy and promising results of immunotherapeutic vaccines have garnered significant attention from both healthcare professionals and patients, driving its substantial market share.
Following closely behind Immunotherapeutic Vaccines, Booster Vaccines emerged as another prominent player in the Tuberculosis Vaccine Treatment Market. These vaccines, designed to enhance and prolong the immunity provided by primary vaccination, accounted for a considerable portion of the market share. With their ability to bolster the body's defense mechanisms against tuberculosis, Booster Vaccines have become integral in reinforcing immunity among individuals at risk of contracting the disease or those requiring additional protection.
The Others category encompassed various vaccine types and alternatives in the Tuberculosis Vaccine Treatment Market. This segment comprised a diverse range of products, including conventional vaccines, novel formulations, and experimental treatments. While not as dominant as Immunotherapeutic and Booster Vaccines, the "Others" category contributed significantly to the market landscape, offering alternative options for tuberculosis prevention and treatment.
By Application Analysis
Hospitals dominated the Tuberculosis Vaccine Treatment Market's "By Application" segment with over 50% share.
In 2023, Hospitals held a dominant market position in the By Application segment of the Tuberculosis Vaccine Treatment Market, capturing more than an 50% share. Hospitals emerged as the primary point of access for tuberculosis diagnosis, treatment, and vaccine administration, serving as crucial hubs for patient care and management. Their extensive infrastructure, specialized medical staff, and comprehensive healthcare services positioned hospitals as the cornerstone of tuberculosis treatment, thereby securing a significant share of the market.
Following Hospitals, Clinics represented another substantial player in the Tuberculosis Vaccine Treatment Market. Clinics offered accessible and specialized healthcare services, catering to individuals seeking tuberculosis diagnosis, treatment, and preventive measures.
Research Institutes constituted a notable segment within the Tuberculosis Vaccine Treatment Market, contributing to advancements in vaccine development, efficacy studies, and treatment protocols. These institutes spearheaded research efforts aimed at enhancing vaccine formulations, exploring novel treatment modalities, and understanding tuberculosis pathogenesis.
The "Others" category encompassed various healthcare providers and organizations involved in tuberculosis treatment and vaccine administration. This segment included governmental health agencies, non-profit organizations, and specialty clinics focusing on infectious diseases.
Key Market Segments
By Type
- Immunotherapeutic Vaccines
- Booster Vaccines
- Others
By Application
- Hospitals
- Clinics
- Research Institutes
- Others
Growth Opportunity
Expansion of Vaccination Programs
The year 2024 presents a promising landscape for the global Tuberculosis (TB) vaccine treatment market, primarily driven by the expansion of vaccination programs worldwide. With increased awareness about the burden of TB and the significance of preventive measures, governments and healthcare organizations are ramping up efforts to implement robust vaccination strategies. This expansion is not limited to high-burden regions but extends across the globe, encompassing both developed and developing nations.
As vaccination programs reach more individuals, particularly in regions with high TB prevalence rates, the demand for TB vaccines is expected to surge. This presents a significant growth opportunity for pharmaceutical companies investing in TB vaccine research and development. Moreover, the integration of TB vaccination into routine immunization schedules in various countries further bolsters market prospects, ensuring sustained demand for TB vaccines in the foreseeable future.
Collaborations to Accelerate Vaccine Development
Collaborations between pharmaceutical companies, research institutions, and governmental bodies are playing a pivotal role in accelerating TB vaccine development. By pooling resources, expertise, and infrastructure, these collaborations expedite the research and clinical trial processes, bringing novel TB vaccine candidates to market more efficiently.
In 2024, strategic partnerships and alliances are poised to catalyze innovation in TB vaccine development, driving the introduction of next-generation vaccines with enhanced efficacy and safety profiles. These collaborative efforts not only foster innovation but also mitigate the financial and technical challenges associated with vaccine development, ultimately benefiting patients and healthcare systems globally.
Latest Trends
Personalized Vaccination Strategies
concept of personalized medicine is gaining traction in TB vaccine development. With advancements in genomics and immunology, researchers are exploring the feasibility of tailoring TB vaccines to individual genetic profiles and immune responses. This approach aims to optimize vaccine efficacy and minimize adverse reactions by identifying and targeting specific host-pathogen interactions. Personalized vaccination strategies have the potential to revolutionize TB prevention by offering tailored solutions for diverse population groups, particularly in regions with high TB burden and varying genetic susceptibilities.
Combination Vaccines and Multi-Dose Regimens
In response to the complex nature of TB infection, there is a growing interest in developing combination vaccines and multi-dose regimens. By targeting multiple TB antigens simultaneously or employing sequential vaccination schedules, these approaches seek to enhance vaccine-induced immunity and provide broader protection against different strains and stages of TB. Moreover, combination vaccines and multi-dose regimens offer logistical advantages, simplifying vaccination programs and improving patient compliance. However, challenges such as vaccine compatibility, safety, and regulatory approval processes need to be addressed to realize the full potential of these innovative strategies.
Regional Analysis
The Asia Pacific region emerges as the dominant force in the Tuberculosis Vaccine Treatment Market, commanding a substantial share of approximately 40%.
Asia Pacific emerges as the dominant force in the Tuberculosis Vaccine Treatment Market, capturing a significant share of approximately 40%. With a staggering disease burden accounting for over 60% of global TB cases, the region faces profound public health challenges. However, rapid economic growth, coupled with increasing healthcare expenditure, propels investments in TB prevention and treatment. Countries such as India, China, and Indonesia spearhead the market, driven by large patient populations and expanding immunization programs.
In North America, the Tuberculosis Vaccine Treatment Market exhibits a mature yet dynamic landscape, characterized by robust research infrastructure and high healthcare expenditure. With a prevalence rate of approximately 3 cases per 100,000 population, the region demonstrates a significant burden of tuberculosis. However, proactive government initiatives, coupled with strong private sector participation, drive innovation and adoption of advanced treatment modalities.
Europe represents a key market for tuberculosis vaccine treatment, marked by a diverse landscape influenced by varying disease burdens and healthcare systems. With an estimated incidence rate of 30 cases per 100,000 population, the region grapples with persistent challenges in TB control despite substantial investments in research and development.
In the Middle East & Africa region, the Tuberculosis Vaccine Treatment Market reflects a diverse landscape shaped by socioeconomic disparities and regional variations in disease prevalence. While tuberculosis incidence rates vary across countries, ranging from 30 to 300 cases per 100,000 population, limited healthcare infrastructure and resource constraints pose significant challenges to effective TB control.
Latin America presents a complex market for tuberculosis vaccine treatment, characterized by a mix of high-burden countries and middle-income economies. With an estimated incidence rate of 25 cases per 100,000 population, the region grapples with persistent challenges in TB control, exacerbated by socioeconomic inequalities and limited access to healthcare services.
Key Regions and Countries
North America
- US
- Canada
- Mexico
Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
In 2024, the global Tuberculosis Vaccine Treatment Market witnesses a dynamic interplay of key players shaping the landscape. Among these influential entities, companies such as Valneva plc, Bavarian Nordic, Sanofi Pasteur, Biofabri, and GlaxoSmithKline stand at the forefront, driving innovation and advancements in TB prevention and treatment. These companies leverage their extensive research and development capabilities, coupled with strategic partnerships and investments, to address the complex challenges posed by tuberculosis.
The market is propelled by prominent players including China National Biotec Group, Serum Institute of India, IDT Biologics, and Japan BCG Lab, each contributing unique expertise and resources to the global fight against TB. These companies play a pivotal role in expanding access to vaccines and treatments, particularly in regions with high disease burden.
Emerging players such as GreenSignal Bio Pharma, Taj Pharmaceuticals, Merck, and Longcom Enterprise Ltd., are poised to make significant contributions to the market, capitalizing on opportunities for innovation and market expansion. These companies bring fresh perspectives and novel approaches to TB vaccine development and manufacturing, driving competition and spurring advancements in the field.
The diverse ecosystem of key players in the Tuberculosis Vaccine Treatment Market underscores the collaborative effort required to address the multifaceted challenges of tuberculosis on a global scale. As these companies continue to invest in research, forge strategic partnerships, and navigate regulatory landscapes, they remain integral to the quest for effective TB prevention and treatment strategies, ultimately contributing to the realization of a TB-free world.
Market Key Players
- Valneva plc
- Bavarian Nordic
- Sanofi Pasture
- Biofabri
- GlaxoSmithKline
- China National Biotec Group
- Serum Institute of India
- IDT Biologics
- Japan BCG Lab
- GreenSignal Bio Pharma
- Taj Pharmaceuticals
- Merck
- Longcom Enterprise Ltd
Recent Development
In February 2024, ImmunityBio partners with Serum Institute to address BCG shortage for bladder cancer treatment. Supplying standard and recombinant BCG for trials, enhancing patient outcomes. ImmunityBio, Inc. and Serum Institute collaborate for cancer treatment advancement.
In March 2024, WHO Released investment case for TB screening and preventive treatment, highlighting benefits and urging increased resources for global TB efforts, aligning with commitments made at 2023 UN High-Level Meeting on TB.
In February 2024, IQVIA, Dr. Almas Shamim stresses TB Preventive Treatment's role in African TB control, urging political commitment, enhanced screening, access to drugs, and effective program management for successful implementation.
Report Scope
Report Features Description Market Value (2023) USD 79.1 Mn Forecast Revenue (2033) USD 107.5 Mn CAGR (2024-2033) 3.2% Base Year for Estimation 2023 Historic Period 2018-2023 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered By Type (Immunotherapeutic Vaccines, Booster Vaccines, Others), By Application (Hospitals, Clinics, Research Institutes, Others) Regional Analysis North America - The US, Canada, & Mexico; Western Europe - Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe - Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC - China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America - Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa - Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Valneva plc, Bavarian Nordic, Sanofi Pasture, Biofabri, GlaxoSmithKline, China National Biotec Group, Serum Institute of India, IDT Biologics, Japan BCG Lab, GreenSignal Bio Pharma, Taj Pharmaceuticals, Merck, Longcom Enterprise Ltd Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Valneva plc
- Bavarian Nordic
- Sanofi Pasture
- Biofabri
- GlaxoSmithKline
- China National Biotec Group
- Serum Institute of India
- IDT Biologics
- Japan BCG Lab
- GreenSignal Bio Pharma
- Taj Pharmaceuticals
- Merck
- Longcom Enterprise Ltd




