
Retrovirus-Based Gene Therapy Drugs Market Report By Type (Lentiviruses, Gamma Retroviruses, Alpha Retroviruses, Beta Retroviruses, Others), By Target Disease (Genetic Disorders, Cancer, Infectious Diseases, Neurological Disorders, Cardiovascular Disorders, Immunodeficiency Disorders, Others), By Delivery Method (Ex Vivo Gene Therapy, In Vivo Gene Therapy), By End Users, By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
-
45889
-
May 2024
-
285
-
-
This report was compiled by Trishita Deb Trishita Deb is an experienced market research and consulting professional with over 7 years of expertise across healthcare, consumer goods, and materials, contributing to over 400 healthcare-related reports. Correspondence Team Lead- Healthcare Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Quick Navigation
Report Overview
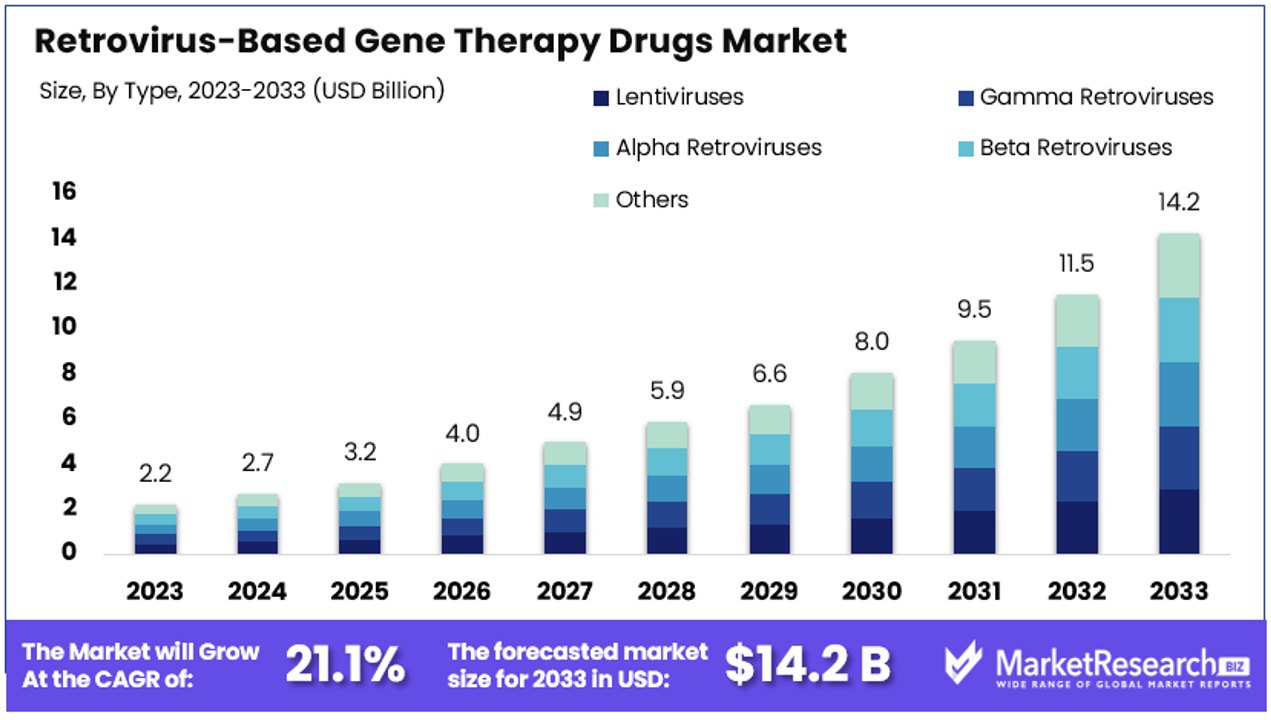
The Global Retrovirus-Based Gene Therapy Drugs Market size is expected to be worth around USD 14.2 Billion by 2033, from USD 2.2 Billion in 2023, growing at a CAGR of 21.1% during the forecast period from 2024 to 2033.
The Retrovirus-Based Gene Therapy Drugs Market encompasses pharmaceuticals derived from retroviruses, aiming to treat genetic disorders by modifying patients' DNA. These therapies leverage retroviral vectors to deliver therapeutic genes into target cells, addressing underlying genetic causes.
With advancements in biotechnology, this market offers promising solutions for diseases with a genetic component, such as certain cancers, immune deficiencies, and genetic disorders. Market projections indicate substantial growth due to ongoing research, regulatory approvals, and increasing investment in gene therapy.
The Retrovirus-Based Gene Therapy Drugs Market presents a compelling landscape within the pharmaceutical sector, driven by its potential to address a broad spectrum of genetic disorders. With over 6,000 known genetic disorders globally, including rare conditions impacting fewer than 200,000 individuals in the U.S. alone, the market offers a substantial addressable patient population. This vast scope underscores the market's significance, as stakeholders seek innovative solutions to meet unmet medical needs.
Advancements in biotechnology have propelled the development of retrovirus-based gene therapy drugs, leveraging retroviral vectors to deliver therapeutic genes into target cells and address underlying genetic anomalies. These therapies hold promise across various indications, ranging from certain cancers to immune deficiencies and rare genetic disorders. The market's growth trajectory is further bolstered by regulatory approvals and ongoing research initiatives, signaling an increasingly favorable environment for investment and development.
Furthermore, the market's dynamics are characterized by a blend of scientific innovation, regulatory frameworks, and market demand. Proactive engagement with key opinion leaders, regulatory bodies, and research institutions is essential to capitalize on growth opportunities and mitigate risks effectively.
The Retrovirus-Based Gene Therapy Drugs Market represents a pivotal segment within the pharmaceutical industry, poised for significant growth and innovation. Stakeholders should maintain a strategic focus on market developments, leveraging data-driven insights and collaboration to drive impactful outcomes and meet the evolving needs of patients worldwide.
Key Takeaways
- Market Value: The Global Retrovirus-Based Gene Therapy Drugs Market is projected to reach USD 14.2 billion by 2033, exhibiting a robust CAGR of 21.1% from 2024 to 2033, significantly propelled by advancements in gene therapy technologies and increasing demand for innovative treatments.
- Type Analysis: Lentiviruses hold the dominant position with a market share of 40%, driven by their high efficiency and broad applicability in treating various genetic disorders and cancers.
- Target Disease Analysis: Genetic disorders emerge as the leading segment, capturing 45% of the market share, owing to the pressing need for effective treatments for prevalent conditions like cystic fibrosis, hemophilia, and muscular dystrophy.
- Delivery Method Analysis: Ex vivo gene therapy dominates with a 60% market share, facilitated by its controlled environment, higher efficiency, and proven success in treating genetic and hematologic disorders.
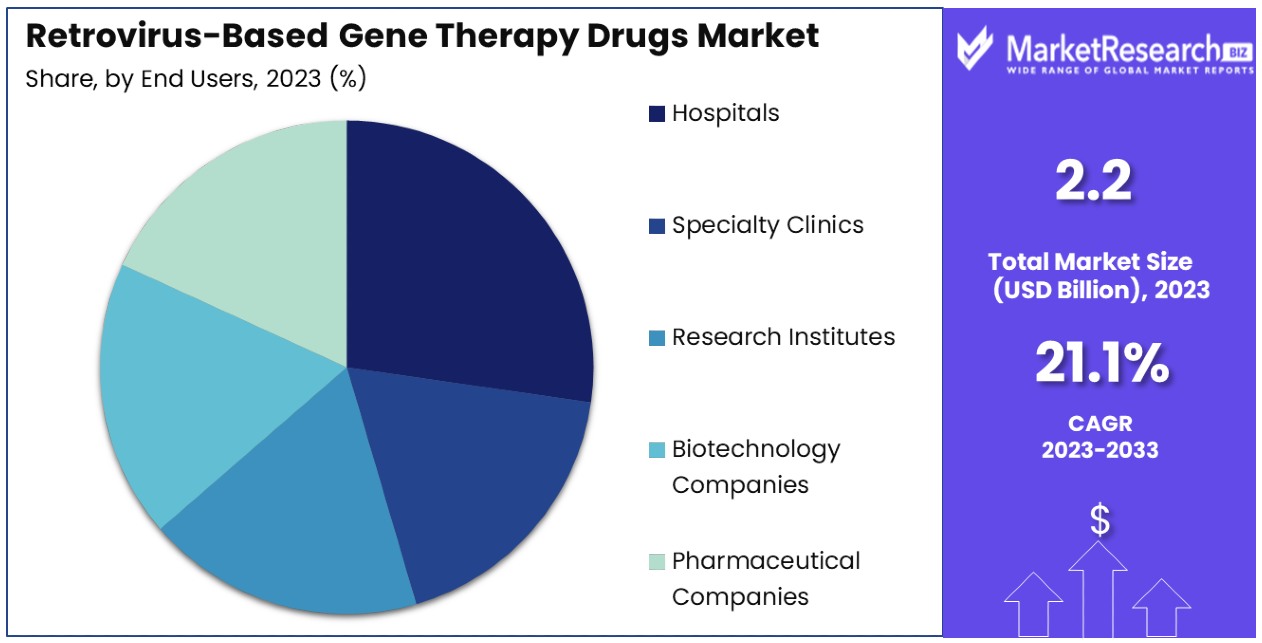
- End Users Analysis: Hospitals lead with a 35% market share, providing accessible and comprehensive care for patients undergoing retrovirus-based gene therapy treatments.
- North America Dominance: North America commands a substantial 40% market share, followed by Europe with a 30% market share, indicating strong market penetration and adoption of retrovirus-based gene therapy drugs in these regions.
- Analyst Viewpoint and Growth Opportunities: Analysts anticipate continued exponential growth in the retrovirus-based gene therapy drugs market, fueled by technological advancements, increasing research investments, and rising demand for personalized medicine solutions. Opportunities lie in expanding therapeutic applications, optimizing delivery methods, and forging strategic partnerships to accelerate market expansion and address unmet medical needs effectively.
Driving Factors
Rising Prevalence of Genetic Disorders Drives Market Growth
The increasing incidence of genetic diseases such as cystic fibrosis, hemophilia, and muscular dystrophy is significantly driving the demand for retrovirus-based gene therapy drugs. These therapies offer a promising approach to treat such conditions by introducing functional genes into the patient's cells. Retrovirus-based gene therapies have shown potential in addressing these disorders by correcting the underlying genetic defects. For example, Strimvelis, a retrovirus-based gene therapy developed by GlaxoSmithKline, is approved for the treatment of adenosine deaminase severe combined immunodeficiency (ADA-SCID), a rare genetic disorder affecting the immune system. The success of Strimvelis demonstrates the efficacy of retrovirus-based therapies and boosts confidence in their application for other genetic conditions.
The rising prevalence of genetic disorders increases the need for innovative treatments, pushing pharmaceutical companies to focus on gene therapy development. As more cases of genetic disorders are diagnosed, the market for retrovirus-based gene therapy drugs is expected to grow. This growth is also supported by advancements in diagnostic technologies that enable early detection and intervention, further expanding the potential patient pool for these therapies.
Advancements in Gene Editing Technologies Drive Market Growth
Rapid progress in gene editing technologies, particularly CRISPR-Cas9, is revolutionizing the development of retrovirus-based gene therapies. These advancements allow for more precise and efficient targeting of genetic sequences, enhancing the potential for effective treatments. Researchers can now modify specific genes with greater accuracy, which is crucial for addressing complex genetic disorders. For instance, researchers at the University of Pennsylvania are exploring the use of CRISPR-Cas9 in conjunction with retroviral vectors to treat sickle cell disease. This combination of cutting-edge technologies promises more effective and durable treatments, thereby increasing the appeal of retrovirus-based therapies.
The integration of gene editing technologies with retroviral vectors accelerates the pace of innovation in the gene therapy field. These advancements also attract significant investments from pharmaceutical companies and research institutions, further driving market growth. As gene editing tools become more refined and accessible, the development of new retrovirus-based therapies is expected to increase, expanding the market and offering new treatment options for patients. The synergy between gene editing advancements and retrovirus-based therapies underscores the potential for transformative healthcare solutions, propelling the market forward.
Increasing Investments in Research and Development Drive Market Growth
Substantial investments in research and development (R&D) from governments, pharmaceutical companies, and biotechnology firms are propelling the retrovirus-based gene therapy drugs market. These investments are crucial for advancing scientific knowledge and developing new therapies. For example, the National Institutes of Health (NIH) in the United States has allocated significant funding for gene therapy research, with a focus on retroviral vectors. Such financial support enables the exploration of innovative approaches and the translation of research findings into clinical applications.
Increased R&D investments lead to a greater understanding of retrovirus-based gene therapies and their potential applications. These funds support preclinical studies, clinical trials, and the development of new therapeutic platforms, accelerating the introduction of effective treatments to the market. As a result, the retrovirus-based gene therapy drugs market benefits from a continuous pipeline of new and improved therapies, enhancing its growth prospects. Furthermore, collaborations between public and private sectors foster innovation and facilitate the commercialization of novel therapies, thereby driving market expansion.
Restraining Factors
Safety Concerns and Potential Side Effects Restrain Market Growth
Safety concerns surrounding retrovirus-based gene therapies pose a significant obstacle to market expansion. Despite advancements, worries persist about adverse effects, such as insertional mutagenesis, which can disrupt gene function and potentially lead to cancer. Past incidents, like the development of leukemia in X-linked severe combined immunodeficiency (X-SCID) patients during clinical trials, have prompted regulatory scrutiny and raised apprehensions among stakeholders.
The market's growth is hampered as these safety concerns deter investment, slow clinical trials, and erode patient confidence. Regulatory agencies impose stringent oversight, prolonging approval processes and increasing development costs. Healthcare professionals hesitate to recommend therapies with uncertain safety profiles, impacting adoption rates. Addressing these safety challenges requires ongoing research, robust risk mitigation strategies, and transparent communication to rebuild trust and foster market growth.
High Costs and Reimbursement Challenges Constrain Market Expansion
High costs associated with developing, manufacturing, and monitoring retrovirus-based gene therapies present formidable barriers to market growth. The intricate nature of these therapies demands substantial resources, translating into exorbitant treatment expenses. Furthermore, reimbursement policies struggle to accommodate the unprecedented pricing of gene therapies like Zolgensma, priced at over $2 million.
The market's expansion is stifled as these costs limit patient access and strain healthcare budgets. Reimbursement challenges hinder adoption rates, as payers grapple with the financial burden of gene therapy. Affordability concerns deter investment and innovation, impeding the development of new therapies. Addressing these challenges necessitates collaborative efforts among stakeholders to establish sustainable pricing models, streamline reimbursement processes, and ensure equitable access to gene therapies, thereby fostering market growth.
Type Analysis
Lentiviruses dominate with 40% due to their high efficiency and broad applicability.
The type segment of the Retrovirus-Based Gene Therapy Drugs Market is primarily driven by lentiviruses, which hold a dominant share of approximately 40%. Lentiviruses are favored due to their high efficiency in transducing non-dividing cells, which makes them suitable for a wide range of therapeutic applications. Their ability to integrate into the host genome ensures stable, long-term expression of the therapeutic gene, which is crucial for the treatment of chronic conditions. The broad applicability of lentiviruses in treating various genetic disorders, cancers, and other diseases further cements their leading position in the market.
Lentiviruses are extensively used in clinical research and have been a cornerstone in the development of several gene therapies. The approval of lentivirus-based therapies, such as Kymriah and Yescarta for cancer treatment, underscores their clinical potential and commercial viability. These therapies have shown significant success in treating specific types of leukemia and lymphoma, highlighting the therapeutic advantages of lentiviral vectors. Additionally, ongoing research and clinical trials are exploring the use of lentiviruses in treating neurological disorders and other complex diseases, which could further expand their market share.
Gamma retroviruses, alpha retroviruses, and beta retroviruses also contribute to the market, albeit to a lesser extent. Gamma retroviruses have been used in early gene therapy trials, particularly for immunodeficiency disorders, but their integration profile poses a risk of insertional mutagenesis. Alpha retroviruses and beta retroviruses are less commonly used but offer potential in specific niche applications. The "Others" category includes emerging retroviral vectors that are being researched for their unique properties and potential advantages over traditional retroviral vectors.
Target Disease Analysis
Genetic Disorders dominate with 45% due to the high prevalence and urgent need for effective treatments.
In the target disease segment, genetic disorders account for the largest share, approximately 45%, of the Retrovirus-Based Gene Therapy Drugs Market. The high prevalence of genetic diseases such as cystic fibrosis, hemophilia, and muscular dystrophy drives the demand for effective gene therapies. Retrovirus-based gene therapies provide a promising solution by correcting the underlying genetic defects, offering long-term relief for patients suffering from these conditions. The successful application of retrovirus-based therapies in treating genetic disorders has been a significant factor in their market dominance.
The increasing incidence of genetic disorders and the limitations of conventional treatments underscore the importance of gene therapy. For example, the approval of Strimvelis for ADA-SCID highlights the effectiveness of retrovirus-based therapies in addressing severe genetic disorders. Moreover, ongoing research and clinical trials are expanding the scope of retrovirus-based therapies to include other genetic conditions, further boosting their market potential. The advancements in gene editing technologies, such as CRISPR-Cas9, are also enhancing the precision and efficacy of these therapies, making them more attractive for treating genetic disorders.
Cancer is another significant segment within the target disease category. Retrovirus-based gene therapies are being developed to target specific cancer types, particularly hematologic malignancies. The success of CAR-T cell therapies, which utilize lentiviral vectors, has demonstrated the potential of retrovirus-based approaches in oncology. Infectious diseases, neurological disorders, cardiovascular disorders, and immunodeficiency disorders are other key segments. While they currently hold smaller market shares, these segments represent areas of active research and development, with several promising therapies in the pipeline.
Delivery Method Analysis
Ex Vivo Gene Therapy dominates with 60% due to controlled environment and higher efficiency.
The delivery method segment of the Retrovirus-Based Gene Therapy Drugs Market is dominated by ex vivo gene therapy, which accounts for approximately 60% of the market share. Ex vivo gene therapy involves the extraction of cells from a patient, genetic modification in a controlled laboratory environment, and subsequent reinfusion of the modified cells back into the patient. This method allows for precise control over the genetic modification process, ensuring higher efficiency and safety. The ability to verify the success of gene transfer and to select the most effectively modified cells before reinfusion contributes to the dominance of ex vivo gene therapy.
Ex vivo gene therapy has shown significant success in treating various genetic and hematologic disorders. For instance, CAR-T cell therapies for cancer treatment utilize ex vivo methods to modify patient T-cells, which are then reintroduced to target and destroy cancer cells. The controlled environment of ex vivo gene therapy reduces the risk of off-target effects and enhances the overall efficacy of the treatment. The expanding applications of ex vivo gene therapy in different therapeutic areas are expected to drive its continued dominance in the market.
In vivo gene therapy, although holding a smaller market share, is gaining traction due to its potential for treating conditions where ex vivo methods are impractical. In vivo gene therapy involves the direct delivery of genetic material into the patient’s body, offering a less invasive alternative to ex vivo methods. This approach is particularly useful for targeting specific tissues or organs, such as the liver or muscles. Advances in vector technology and delivery mechanisms are improving the efficiency and safety of in vivo gene therapy, making it an important area of research and development.
End Users Analysis
Hospitals dominate with 35% due to accessibility and comprehensive care.
The end users segment of the Retrovirus-Based Gene Therapy Drugs Market is led by hospitals, which account for approximately 35% of the market share. Hospitals play a crucial role in the administration of gene therapies, providing the necessary infrastructure and expertise for comprehensive patient care. The accessibility of hospitals and their ability to offer a wide range of medical services make them the preferred choice for gene therapy administration. Hospitals are equipped with advanced medical facilities, ensuring the safe and effective delivery of retrovirus-based gene therapies to patients.
Hospitals are at the forefront of clinical trials and the implementation of new therapies, contributing to their dominance in the market. The involvement of hospitals in gene therapy research and their collaboration with biotechnology and pharmaceutical companies facilitate the translation of innovative treatments into clinical practice. The comprehensive care provided by hospitals, including patient monitoring and management of potential side effects, ensures the optimal use of gene therapies. This holistic approach enhances patient outcomes and supports the growth of the retrovirus-based gene therapy drugs market.
Specialty clinics, research institutes, biotechnology companies, and pharmaceutical companies are also key end users. Specialty clinics focus on specific therapeutic areas and provide targeted care, contributing to the adoption of gene therapies in niche markets. Research institutes play a critical role in the early stages of gene therapy development, conducting fundamental research and preclinical studies. Biotechnology and pharmaceutical companies are involved in the commercial development and distribution of gene therapies, driving market expansion through innovation and collaboration. While these segments currently hold smaller market shares compared to hospitals, their contributions are vital for the overall growth of the market.
Key Market Segments
By Type
- Lentiviruses
- Gamma Retroviruses
- Alpha Retroviruses
- Beta Retroviruses
- Others
By Target Disease
- Genetic Disorders
- Cancer
- Infectious Diseases
- Neurological Disorders
- Cardiovascular Disorders
- Immunodeficiency Disorders
- Others
By Delivery Method
- Ex Vivo Gene Therapy
- In Vivo Gene Therapy
By End Users
- Hospitals
- Specialty Clinics
- Research Institutes
- Biotechnology Companies
- Pharmaceutical Companies
Growth Opportunities
Expansion into Emerging Markets Offers Growth Opportunity
Expansion into emerging markets presents a significant growth opportunity for retrovirus-based gene therapy drugs, driven by the rising demand for innovative treatments and the prevalence of genetic disorders in these regions. Statistics indicate that emerging markets, particularly in Asia and Latin America, face a substantial burden of genetic diseases, yet access to advanced therapies remains limited.
By strategically entering these markets through partnerships, technology transfer, and local manufacturing, companies can capitalize on untapped opportunities and expand their market presence. For example, companies like Bluebird Bio and Orchard Therapeutics are actively exploring entry into these regions, recognizing the potential for growth and the need for accessible treatments in underserved communities. This expansion not only addresses unmet medical needs but also fosters sustainable market growth by tapping into diverse patient populations and healthcare ecosystems.
Development of Combination Therapies Offers Growth Opportunity
The development of combination therapies presents a compelling growth opportunity within the Retrovirus-Based Gene Therapy Drugs Market, offering synergistic approaches to complex diseases and expanding therapeutic possibilities. Combining retrovirus-based gene therapies with other modalities such as small molecule drugs, biologics, or cell therapies has the potential to enhance treatment efficacy and broaden the scope of applications.
Recent research focuses on combining retrovirus-based gene therapies with immune checkpoint inhibitors for cancer treatment, aiming to harness the immune system's anti-tumor response. These combination approaches not only address multiple disease pathways but also offer personalized treatment options tailored to individual patient needs. As such, the development of combination therapies not only drives innovation within the market but also unlocks new avenues for therapeutic intervention, paving the way for sustained growth and improved patient outcomes.
Trending Factors
Personalized Medicine Approach: A Trending Factor in the Retrovirus-Based Gene Therapy Drugs Market
The personalized medicine approach is gaining momentum within the Retrovirus-Based Gene Therapy Drugs Market, driven by the shift towards tailored treatments based on individual patient characteristics. Retrovirus-based gene therapies offer a promising avenue for personalization, as viral vectors can be engineered to carry specific therapeutic genes suited to a patient's genetic profile. This trend reflects the industry's focus on enhancing treatment efficacy and minimizing adverse effects by targeting therapies to patients' unique genetic makeup.
Recent statistics indicate a growing interest in personalized gene therapies, with companies like Pfizer and Spark Therapeutics pioneering personalized treatments for rare genetic disorders using retroviral vectors. As personalized medicine continues to garner attention and support from both patients and regulatory agencies, retrovirus-based gene therapy drugs are poised to play a pivotal role in advancing this transformative approach to healthcare.
Focus on Rare Genetic Diseases: A Trending Factor in the Retrovirus-Based Gene Therapy Drugs Market
The increasing focus on rare genetic diseases represents a notable trend within the Retrovirus-Based Gene Therapy Drugs Market, driven by the recognition of significant unmet medical needs and the potential for lucrative market opportunities. While retrovirus-based gene therapies were initially developed for more common genetic disorders, there is a growing emphasis on addressing rare and ultra-rare diseases with limited treatment options.
Regulatory agencies may offer incentives and accelerated pathways for therapies targeting rare diseases, further fueling interest in this area. Companies like Orchard Therapeutics and Freeline Therapeutics are at the forefront of this trend, developing retrovirus-based gene therapies for rare conditions such as metachromatic leukodystrophy and Fabry disease. As research and development efforts intensify, retrovirus-based gene therapy drugs are poised to make significant strides in addressing the unmet needs of patients with rare genetic diseases, driving market growth and innovation.
Regional Analysis
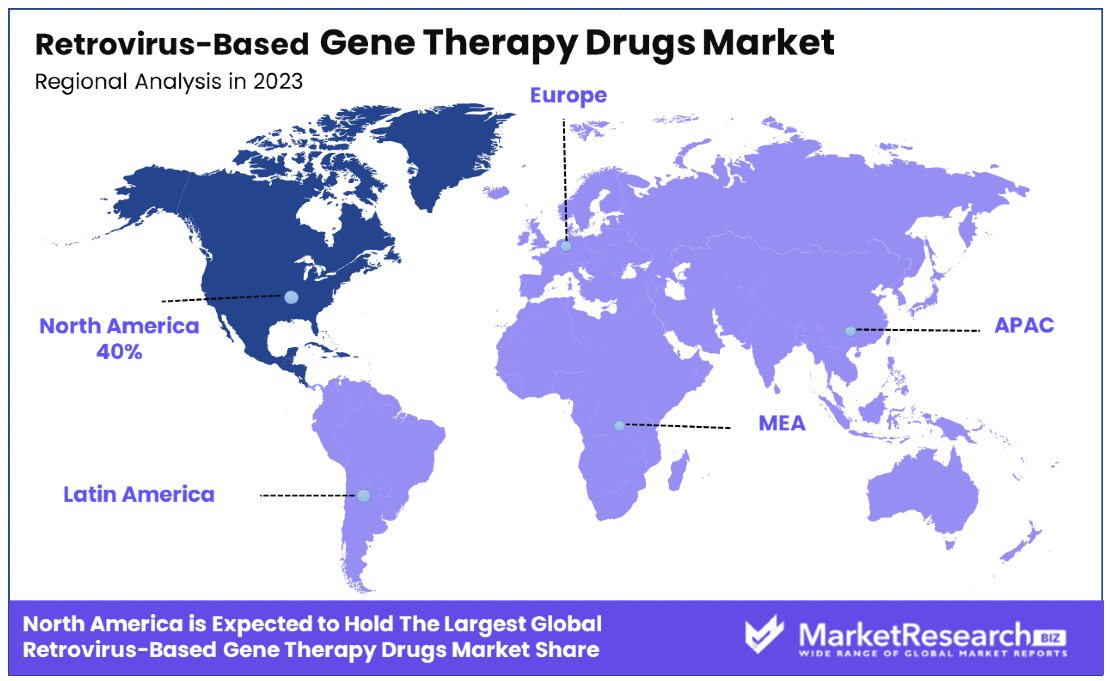
North America Dominates with 40% Market Share
North America's dominance in the Retrovirus-Based Gene Therapy Drugs Market can be attributed to several key factors. Firstly, the region boasts advanced healthcare infrastructure and research capabilities, fostering innovation and driving the development of gene therapies. Additionally, strong regulatory frameworks and favorable reimbursement policies incentivize investment in the market. Furthermore, robust collaborations between academia, biotech companies, and pharmaceutical giants facilitate the translation of research into commercial products, solidifying North America's position as a leader in gene therapy.
North America's high market share is further bolstered by its affluent patient population and increasing prevalence of genetic disorders. The region's well-established healthcare systems ensure widespread access to innovative treatments, driving demand for retrovirus-based gene therapy drugs. Moreover, strategic partnerships between industry players and healthcare providers enhance market penetration and accelerate adoption rates. Additionally, North America's proactive approach to addressing rare diseases aligns with the growing trend towards personalized medicine, further driving market growth and dominance.
Regional Market Shares:
Europe maintains a substantial 30% market share, reflecting its advanced healthcare systems and strong emphasis on research and development in gene therapy.
The Asia Pacific region holds a noteworthy 20% market share, driven by increasing healthcare expenditure and growing awareness of gene therapy's potential in addressing genetic disorders.
The Middle East & Africa region accounts for a modest 5% market share, indicating a nascent but emerging interest in retrovirus-based gene therapy drugs.
Latin America also holds a 5% market share, indicating a growing demand for innovative treatments amidst improving healthcare infrastructure and rising prevalence of genetic disorders.
Key Regions and Countries
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
The Retrovirus-Based Gene Therapy Drugs Market is characterized by a dynamic landscape shaped by key players driving innovation, research, and commercialization efforts. Leading the forefront of this market are prominent pharmaceutical and biotechnology companies, each contributing uniquely to market advancement.
Novartis AG stands as a frontrunner in the market, leveraging its extensive resources and expertise to develop groundbreaking gene therapies. Similarly, bluebird bio has established itself as a key player, focusing on personalized medicine approaches and pioneering treatments for rare genetic disorders.
The acquisition of Kite Pharma by Gilead Sciences has positioned the company as a major contender in the gene therapy space, particularly in the field of cancer immunotherapy. Likewise, Spark Therapeutics, under the umbrella of Roche, brings substantial resources and global reach to drive the commercialization of gene therapies.
Other notable players include Sangamo Therapeutics, uniQure N.V., and Voyager Therapeutics, each contributing innovative gene therapy solutions targeting various diseases. Additionally, companies like Rocket Pharmaceuticals, Avrobio, and Orchard Therapeutics are actively engaged in advancing gene therapy research and development, particularly for rare genetic diseases.
Furthermore, GenSight Biologics, Takara Bio Inc., and Genethon play significant roles in advancing gene therapy technologies and applications. Renova Therapeutics and Precision BioSciences also contribute to the market with their unique therapeutic approaches and cutting-edge research initiatives.
Collectively, these key players drive market growth through strategic partnerships, clinical advancements, and regulatory approvals, shaping the future landscape of the Retrovirus-Based Gene Therapy Drugs Market. Their continued efforts in research, development, and commercialization pave the way for transformative treatments that address unmet medical needs and improve patient outcomes.
Market Key Players
- Novartis AG
- bluebird bio
- Kite Pharma (a subsidiary of Gilead Sciences)
- Spark Therapeutics (a subsidiary of Roche)
- Sangamo Therapeutics
- uniQure N.V.
- Voyager Therapeutics
- Rocket Pharmaceuticals
- Avrobio
- Orchard Therapeutics
- GenSight Biologics
- Takara Bio Inc.
- Genethon
- Renova Therapeutics
- Precision BioSciences
Recent Developments
- In February 2024, a rare case of retrovirus integration was discovered in a rodent from New Guinea, the white-bellied mosaic-tailed rat. This recent case of retrovirus colonisation sheds light on the process of genome integration, a phenomenon where retroviruses incorporate their genes into the host genome.
- On April 2024, research reveals that an ancient retrovirus embedded in the DNA of jawed vertebrates plays a crucial role in the evolution of speedy nerves. This retrovirus, known as RetroMyelin, aids in the production of a protein necessary for insulating nerve fibers, contributing to the development of myelin.
- On April 2024, a study by researchers at the Spanish National Cancer Research Centre (CNIO) uncovered the role of ancient viral remnants in embryo development. These remnants, known as endogenous retroviruses (ERVs), have integrated into the host genome over millions of years.
Report Scope
Report Features Description Market Value (2023) USD 2.2 Billion Forecast Revenue (2033) USD 14.2 Billion CAGR (2024-2033) 21.1% Base Year for Estimation 2023 Historic Period 2018-2023 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered By Type (Lentiviruses, Gamma Retroviruses, Alpha Retroviruses, Beta Retroviruses, Others), By Target Disease (Genetic Disorders, Cancer, Infectious Diseases, Neurological Disorders, Cardiovascular Disorders, Immunodeficiency Disorders, Others), By Delivery Method (Ex Vivo Gene Therapy, In Vivo Gene Therapy), By End Users (Hospitals, Specialty Clinics, Research Institutes, Biotechnology Companies, Pharmaceutical Companies) Regional Analysis North America - The US, Canada, & Mexico; Western Europe - Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe - Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC - China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America - Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa - Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Novartis AG, bluebird bio, Kite Pharma (a subsidiary of Gilead Sciences), Spark Therapeutics (a subsidiary of Roche), Sangamo Therapeutics, uniQure N.V., Voyager Therapeutics, Rocket Pharmaceuticals, Avrobio, Orchard Therapeutics, GenSight Biologics, Takara Bio Inc., Genethon, Renova Therapeutics, Precision BioSciences Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Novartis AG
- bluebird bio
- Kite Pharma (a subsidiary of Gilead Sciences)
- Spark Therapeutics (a subsidiary of Roche)
- Sangamo Therapeutics
- uniQure N.V.
- Voyager Therapeutics
- Rocket Pharmaceuticals
- Avrobio
- Orchard Therapeutics
- GenSight Biologics
- Takara Bio Inc.
- Genethon
- Renova Therapeutics
- Precision BioSciences




