
Personalized Medicine Biomarkers Market By Application(Treatment Selection, Early Detection/Screening, Diagnosis, Monitoring), By Indication(Oncology, Neurology, Diabetes, Cardiology, Others), By Region And Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, And Forecast 2023-2032
-
42398
-
Dec 2023
-
179
-
-
This report was compiled by Correspondence Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Quick Navigation
- Personalized Medicine Biomarkers Market Size, Share, Trends Analysis
- Personalized Medicine Biomarkers Market Dynamics
- Personalized Medicine Biomarkers Market Segmentation Analysis
- Personalized Medicine Biomarkers Industry Segments
- Personalized Medicine Biomarkers Market Growth Opportunities
- Personalized Medicine Biomarkers Market Regional Analysis
- Personalized Medicine Biomarkers Industry By Region
- Personalized Medicine Biomarkers Market Share Analysis
- Personalized Medicine Biomarkers Industry Key Players
- Personalized Medicine Biomarkers Market Recent Development
- Report Scope
The global Personalized Medicine Biomarkers Market was valued at USD 15.5 Billion in 2022. It is expected to reach USD 67.6 Billion by 2032, with a CAGR of 16.3% during the forecast period from 2023 to 2032.
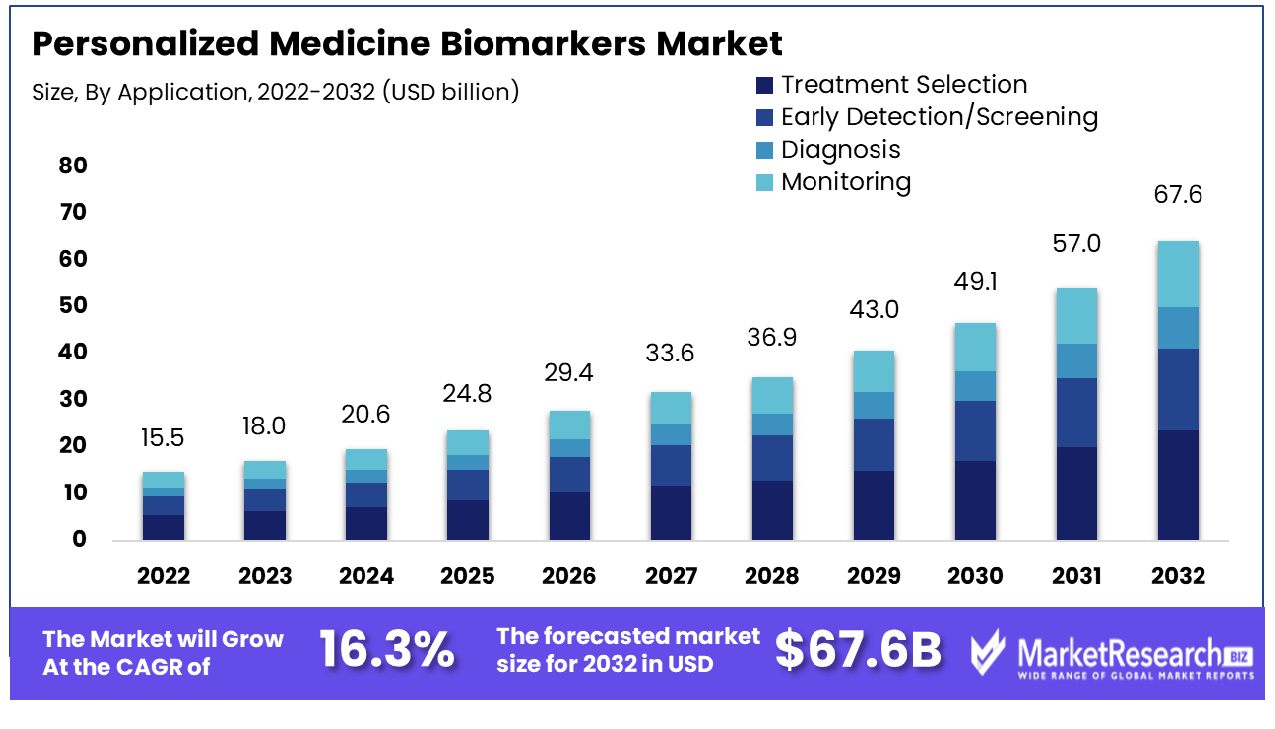
Personalized medicine biomarkers are biological indicators that provide information about an individual's genetic composition and molecular processes. By analyzing specific biomarkers such as genes, proteins, or metabolites for any given patient, healthcare providers can personalize diagnosis, treatment, and prevention plans accordingly.
Individualized approaches result in superior clinical results because treatments target each individual's specific molecular underpinnings of disease rather than using one-size-fits-all methods. Prognostic allow medications and therapies to be adapted to maximize efficacy based on the molecular profile of each patient. Implementation of molecular marker-guided personalized strategies promises to revolutionize medicine.
The FDA has listed more than 160 pharmacogenomic biomarkers and molecular marker signatures, with oncology having 33.5% and neurology having 6.1%. These prognostic biomarkers help identify the most effective treatments for individual patients, improving their clinical diagnostics.
The growth of the personalized medicine biomarkers market is being driven by several key factors. Advances in genomics and proteomics are allowing for the identification of new prognostic that can help predict disease risk and treatment response on an individual level.
For Instance, In September 2023, a new method was developed by scientists at Imperial College London in collaboration with Oxford Nanopore Technologies, involving nanopore sequencing and DNA barcoding. This method has the potential to significantly impact personalized medicine by transforming testing for conditions such as heart disease and cancer.
Additionally, the declining costs and improved accuracy of genomic sequencing are making personalized medicine more accessible and practical.
PETNET Solutions, a company under Siemens Healthineers, is fortifying the foundation for personalized medicine by investing in its network to increase patient access to PET (positron emission tomography) prognostic and support future physiological marker development. The company is upgrading its network of cyclotron-equipped radiopharmacies for PET radiopharmaceuticals with new production facilities.
Other drivers include the rising incidence of various diseases like cancer, autoimmune disorders, and cardiovascular diseases which require more tailored treatment approaches. Pharmaceutical companies are also investing heavily in companion diagnostics and targeted therapies as they promise higher efficacy and safety. Growing consumer demand for personalized approaches instead of the one-size-fits-all model is fostering innovation in this space. These factors eventually propel the personalized medicine biomarkers market growth.
Cell Signalling Technology and Leica Biosystems are collaborating on companion diagnostics (CDx) for personalized medicine. CST provides validated antibodies for immunohistochemistry (IHC), and this partnership supports the development of diagnostic solutions for identifying patients who would benefit from new therapies. The collaboration facilitates the use of CST antibodies by Leica Biosystems' CDx team in clinical diagnostics, aiding in patient recruitment and disease marker status assessment for personalized treatment decisions.
Additionally, supportive government policies and funding for research in personalized medicine are expected to boost disease marker discovery and clinical validation. Overall, the market is poised for rapid fastest growth thanks to powerful demographic shifts, technological improvements, and medical needs. Key challenges will include demonstrating clinical utility and cost-effectiveness at a larger scale.
Personalized Medicine Biomarkers Market Dynamics
Customized Medicine and Chronic Illness Tracking Spur Biomarker Market
The growing demand for personalized medicine and technical advancements in the identification and tracking of chronic diseases are significantly driving the growth of the Personalized Medicine Biomarkers Market. The purpose of individualized medicine is to provide a customized treatment to meet the individual patients' needs by using biomarkers to ensure the diagnosis is correct and precise treatment.
Technology advancements particularly in bioinformatics and genomics have made it simpler to identify and monitor biomarkers related to chronic diseases. This is an indication of a shift in healthcare toward more customized treatments, which has led to a greater demand for biomarkers that provide precise details on particular health conditions.
Rising Cancer and Cardiac Issues Boost Companion Diagnostics
The growing prevalence of cardiac and cancer which has led to the increasing significance of diagnostic tests for companions is one of the major factors in the development of Personalized Medicine Biomarkers Market. The tests are performed to assess the effectiveness of a particular treatment for a patient, usually determined by the presence of biomarkers specific to.
As the number of cancer patients increases and heart conditions, the requirement for precise, individualized treatments becomes crucial. Biomarkers play an essential role in companion diagnosis, which helps healthcare professionals select the most efficient treatments that will benefit their patients. This trend is expected to continue because of the rising number of diseases that are being diagnosed and the growing need for targeted treatment that will drive the need for disease marker technologies.
Biomarkers' Role in Diagnosis and Drug Research Amplifies Market
The growing use of biomarkers in disease diagnosis, companion diagnostics, illness risk evaluation, and drug research is profoundly impacting the Personalized Medicine Biomarker industry. Biomarkers are increasingly being utilized for early disease detection, assessing disease risk, and monitoring treatment responses.
Their importance in drug development and research especially in identifying possible therapeutic targets and assessing the efficacy of drugs, is crucial. Clinical marker's wide-ranging application has transformed the field of pharmaceutical and healthcare research which makes them vital instruments. The increasing reliance on prognostic across various aspects of healthcare and drug development suggests a market poised for sustained growth, as they continue to play a crucial role in advancing personalized medicine and improving patient outcomes.
Measurement Errors of Biomarkers Restrain Personalized Medicine Biomarkers Market Growth
The accuracy and reliability of diagnostic marker measurements are critical in personalized medicine, and errors in these measurements significantly limit the market's growth. Prognostics are used to guide personalized treatment decisions, and any inaccuracies can lead to ineffective or even harmful treatments.
Measurement errors can arise from various factors, including technological limitations, variability in sample handling, and biological factors like patient heterogeneity. These errors undermine the credibility and effectiveness of personalized medicine, making healthcare providers and patients hesitant to rely on prognostic marker-based treatments. Ensuring precise and consistent diagnostic marker measurements is crucial for the expansion and acceptance of personalized medicine.
Data Privacy and Security Concerns Limit Personalized Medicine Biomarkers Market Growth
Data privacy and security concerns are major barriers to the growth of the personalized medicine biomarkers market. The basis of personalized medicine is the analysis and collection of sensitive patient information such as genetic information, that raises privacy concerns.
The threat of unauthorized access to this information is a significant issue for health professionals alike. Adherence to strict data protection regulations and ensuring robust cybersecurity measures are essential, yet challenging and costly. These privacy and security challenges can deter the adoption of personalized medicine practices, as trust in the confidentiality and integrity of patient data is crucial in healthcare.
Personalized Medicine Biomarkers Market Segmentation Analysis
By Application Analysis
Treatment selection is the primary application in the Personalized Medicine Biomarkers Market. This segment’s prominence is driven by the increasing emphasis on personalized therapy, particularly in oncology, where biomarkers guide the use of targeted therapies. Prognostics play a significant function in determining the patients most likely to be benefiting from specific treatments, which may improve the efficacy of treatments and reduce adverse side consequences.
Recent advancements on the subject of proteomics and genomics have significantly enriched the field of prognostic and resulted in a more precise and effective treatment selection. The expanding incorporation of AI, as well as machine learning into diagnostic marker analysis, also improves the accuracy and effectiveness of treatment decision-making.
Biomarkers are increasingly used for early detection/screening, allowing for timely intervention in diseases like cancer. In diagnosis, they help in accurately identifying specific disease types. Monitoring biomarkers is crucial in tracking disease progression and treatment response. Each of these applications is essential for advancing personalized medicine, contributing to the market's growth and diversification.
By Indication Analysis
Oncology is the leading indication in the Personalized Medicine Biomarkers Market. The segment’s dominance is attributed to the critical role biomarkers play in cancer treatment, from diagnosis to therapy selection and monitoring. Predictive markers that are developed that are used in oncology, such as the HER2 gene that is found in breast cancer and EGFR modifications in lung cancer, have changed the way cancer patients are treated, resulting in safer and more effective treatments. The study and the development of novel biomarkers for cancer continue to accelerate progress and development in this area.
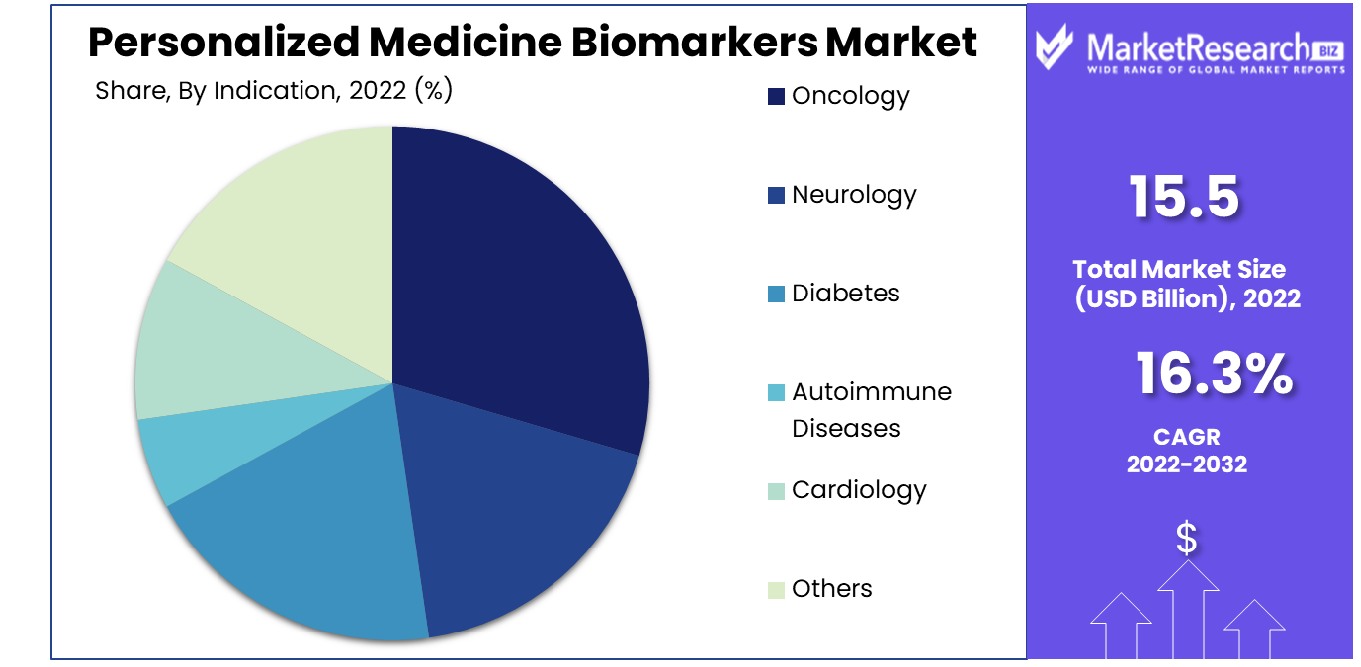
Neurology is a field that has been growing rapidly biomarkers are emerging for diseases such as Alzheimer's disease and Parkinson's disease. Diabetes predictive markers assist in the management of disease and preventative strategies. Autoimmune disease and cardiology also benefit from biomarkers for disease characterization and treatment monitoring. The 'Others' category includes various diseases where biomarkers are increasingly being recognized for their potential in personalized treatment approaches.
Personalized Medicine Biomarkers Industry Segments
By Application
- Treatment Selection
- Early Detection/Screening
- Diagnosis
- Monitoring
By Indication
- Oncology
- Neurology
- Diabetes
- Autoimmune Diseases
- Cardiology
- Others
Personalized Medicine Biomarkers Market Growth Opportunities
Increased Investment in R&D Offers Growth Opportunity in Personalized Medicine Biomarkers Market
The increasing amount of money invested in the field of research and development (R&D) within the area of personalized medicine has been a major reason for the rapid growth of the market for biomarkers. More funding has enabled the development of more thorough studies into biological indicator discovery and validation, which is leading to advances in precision medicine.
These investments are crucial for developing new diagnostic tools and targeted therapies based on individual patient profiles. Recent biomarkers market trends indicate that both public and private sectors are boosting their R&D spending in personalized medicine, reflecting a strong market expansion potential for biomarkers, which are fundamental in tailoring individualized treatment plans.
Emerging Economies Fuel Growth in Personalized Medicine Biomarkers Market
The burgeoning healthcare sector in emerging economies presents a substantial growth opportunity for the personalized medicine biomarkers market. These regions are witnessing rapid growth in the healthcare infrastructure, a rise in expenditure on healthcare, and an increasing awareness of personalized treatment strategies. As economies continue to grow and expand, there is a growing need for medical breakthroughs that comprise personalized medicine based on biomarkers for the effective treatment of illnesses. The expanding capabilities of medical technology in these areas, and the increasing middle class, suggest that there is a great possibility of expanding and utilizing personalized medicine that is based on predictive markers.
Personalized Medicine Biomarkers Market Regional Analysis
North America Dominates with a 36.10% Market Share
North America's leading 36.10% share in the Personalized Medicine Biomarkers Market can be attributed to its advanced healthcare and biotechnology sectors. The region, particularly the United States, is at the forefront of genomic and proteomic research, crucial for biological indicator development. The strong emphasis on personalized medicine and targeted therapies in healthcare also drives this market dominance.
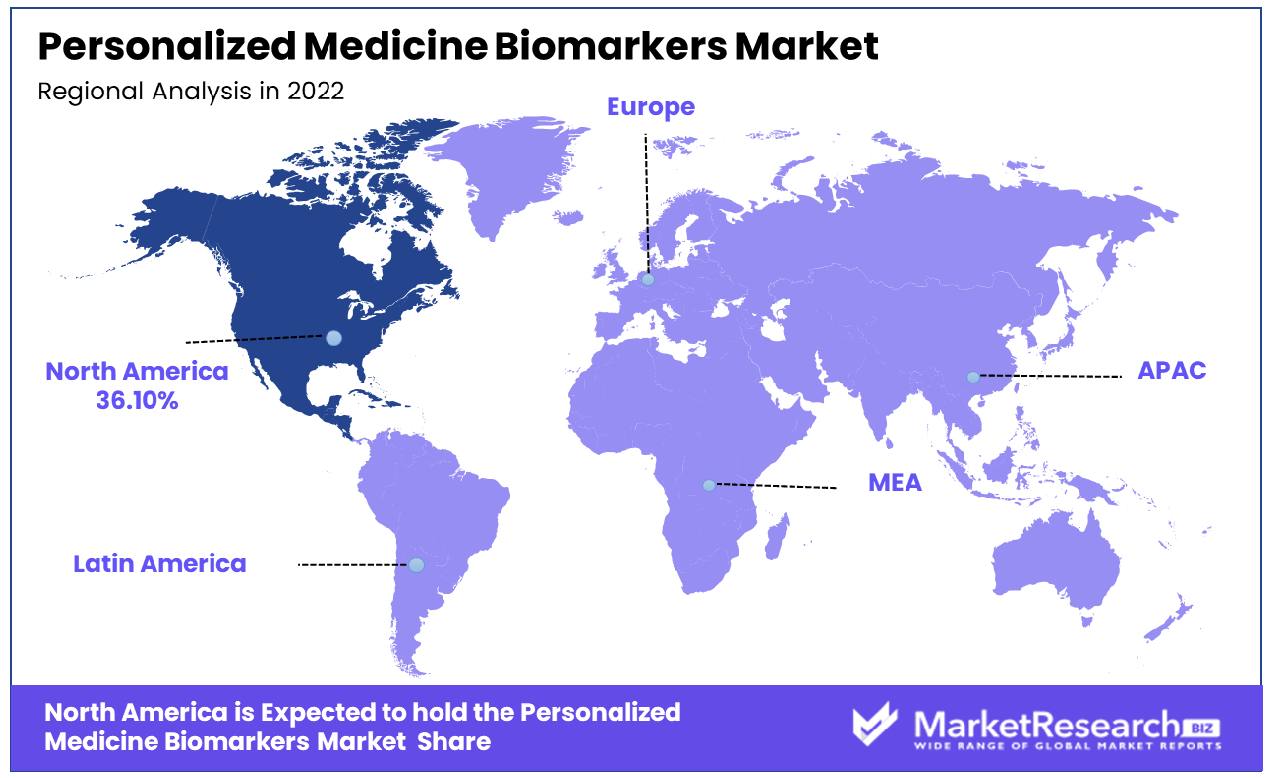
The market dynamics in North America are influenced by a collaborative ecosystem involving academic institutions, healthcare providers, and the biotech industry, fostering innovation in biological marker discovery. The region’s regulatory environment, particularly the FDA’s support for personalized medicine, plays a key role in accelerating the development and approval of biochemical marker-based tests.
Looking ahead, North America’s influence in the personalized medicine biomarkers market is expected to grow. The increasing focus on patient-specific treatment approaches and advancements in genomic sequencing technologies are likely to drive market expansion. The region’s ongoing commitment to healthcare innovation and the trend toward healthcare data digitization and analysis will continue to propel the development of new biomarkers, sustaining North America’s market leadership.
Europe Has Strong Research Infrastructure and Policy Support
Europe’s personalized medicine biomarkers market benefits from its strong research infrastructure and supportive healthcare policies. The region’s commitment to healthcare innovation, along with collaborative research initiatives across EU countries, fosters the development of biomarkers. Europe’s focus on integrating personalized medicine into healthcare systems and the emphasis on regulatory frameworks for biochemical marker validation and use contribute to the market's growth.
Asia-Pacific's Rapid Growth and Increasing Healthcare Investments
The Asia-Pacific region's personalized medicine biomarkers market is experiencing rapid growth, driven by increasing healthcare investments and the growing prevalence of diseases like cancer. Countries such as China and Japan are investing in genomic research, which is pivotal for biochemical marker development. The region's expanding healthcare infrastructure and rising middle class, along with a growing focus on advanced medical treatments, present significant growth opportunities for the personalized medicine market.
Personalized Medicine Biomarkers Industry By Region
North America
- The US
- Canada
- Rest of North America
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherlands
- Rest of Europe
Asia-Pacific
- China
- Japan
- South Korea
- India
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of Asia Pacific
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of Middle East & Africa
Personalized Medicine Biomarkers Market is a rapidly evolving segment at the forefront of precision healthcare, the listed companies are making significant strides. Siemens Healthineers, Roche, and Abbott Laboratories are pivotal in this sector, providing advanced diagnostic technologies and a broad range of disease marker tests that are crucial for personalized treatment strategies. Their global presence and R&D investments significantly influence market trends and standards.
Nexus-DX and Qiagen excel in offering innovative diagnostic solutions and assays that enhance predictive marker identification, reflecting the market's shift towards targeted and individualized diagnostic approaches. PerkinElmer and Lifesign LLC, with their focus on specialized diagnostic technologies, cater to the rising demand for precision medicine and personalized healthcare solutions.
Merck & Co Inc. and Bio-Rad Laboratories, renowned for their contributions to pharmaceuticals and life sciences, are instrumental in integrating biological indicator research into drug development and clinical applications. EKF Diagnostics Holdings plc and Singulex Inc., though smaller in comparison, contribute significantly with niche offerings that underscore the industry's diversity and the growing need for high-sensitivity clinical marker detection.
Signosis Inc. and BioSims Technologies, focusing on cutting-edge diagnostic and analytical tools, represent the market's innovative edge, developing solutions for complex biological indicator analysis. Agilent Technologies and Thermo Fisher Scientific, Inc., with their extensive range of scientific instruments and reagents, play key roles in supporting the underlying technology and research infrastructure necessary for the development and application of personalized medicine biomarkers.
Collectively, these companies not only drive the Personalized Medicine disease marker Market's growth but also represent a spectrum of strategies, from advancing diagnostic technologies to pioneering research in biological indicator applications, crucial for shaping the future of personalized healthcare.
Personalized Medicine Biomarkers Industry Key Players
- Siemens Healthineers
- F. Hoffmann-La Roche Ltd
- Abbott Laboratories
- Nexus-DX
- Qiagen
- PerkinElmer Inc.
- Lifesign LLC
- Merck & Co Inc.
- Bio-Rad Laboratories, Inc.
- EKF Diagnostics Holdings plc
- Singulex Inc.
- Signosis Inc.
- BioSims Technologies
- Agilent Technologies, Inc.
- Thermo Fisher Scientific, Inc.
- Illumina Inc.
- Myriad Genetics, Inc.
- Biomarker Technologies, Inc.
- Danaher Corporation
- Epigenomics AG
Personalized Medicine Biomarkers Market Recent Development
- In November 2023, Engine Biosciences announced the completion of a US$27 million Series A extension funding round. The funds will be used to advance biomarkers and target discoveries through collaboration.
- In September 2023, Wake Forest University School of Medicine was awarded $1.9 million in funding by the National Institute on Drug Abuse (NIDA) to support their research in the field of biomarkers for chronic pain. Chronic pain is a debilitating condition affecting millions of individuals and has a significant impact on their quality of life.
- In 2023, Pitango HealthTech announced the first closing of its second dedicated healthcare fund, Pitango HealthTech II. This fund is valued at $175 million and is specifically aimed at investing in healthcare innovation, including personalized medicine.
- In 2023, LUNGevity and the Moffitt Cancer Center initiated a collaborative effort to address disparities in biological indicator testing access for Black patients with non-small cell lung cancer (NSCLC). The primary focus of this partnership is to quantitatively assess and enhance the availability of disease marker testing for this specific patient population.
Report Scope
Report Features Description Market Value (2022) USD 15.5 Billion Forecast Revenue (2032) USD 67.6 Billion CAGR (2023-2032) 16.3% Base Year for Estimation 2022 Historic Period 2016-2022 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Application(Treatment Selection, Early Detection/Screening, Diagnosis, Monitoring), By Indication(Oncology, Neurology, Diabetes, Autoimmune Diseases, Cardiology, Others) Regional Analysis North America - The US, Canada, Rest of North America, Europe - Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe, Asia-Pacific - China, Japan, South Korea, India, New Zealand, Singapore, Thailand, Vietnam, Rest of Asia Pacific, Latin America - Brazil, Mexico, Rest of Latin America, Middle East & Africa - South Africa, Saudi Arabia, UAE, Rest of Middle East & Africa Competitive Landscape Siemens Healthineers, F. Hoffmann-La Roche Ltd, Abbott Laboratories, Nexus-DX, Qiagen, PerkinElmer Inc., Lifesign LLC, Merck & Co Inc., Bio-Rad Laboratories, Inc., EKF Diagnostics Holdings plc, Singulex Inc., Signosis Inc., BioSims Technologies, Agilent Technologies, Inc., Thermo Fisher Scientific, Inc., Illumina Inc., Myriad Genetics, Inc., Biomarker Technologies, Inc., Danaher Corporation, Epigenomics AG Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Siemens Healthineers
- F. Hoffmann-La Roche Ltd
- Abbott Laboratories
- Nexus-DX
- Qiagen
- PerkinElmer Inc.
- Lifesign LLC
- Merck & Co Inc.
- Bio-Rad Laboratories, Inc.
- EKF Diagnostics Holdings plc
- Singulex Inc.
- Signosis Inc.
- BioSims Technologies
- Agilent Technologies Inc.
- Thermo Fisher Scientific, Inc.
- Illumina Inc.
- Myriad Genetics, Inc.
- Biomarker Technologies, Inc.
- Danaher Corporation
- Epigenomics AG




