
Krabbe Disease Treatment Market By Type of Treatment(Anticonvulsant Medication, Physical Therapy, Muscle Relaxer Drugs, Bone Marrow Transplantation), By End Users(Hospitals and Clinics, Research Centers, Laboratories, Others), By Region And Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, And Forecast 2024-2033
-
42709
-
Jan 2024
-
179
-
-
This report was compiled by Correspondence Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Krabbe Disease Treatment Market Size, Share, Trends Analysis
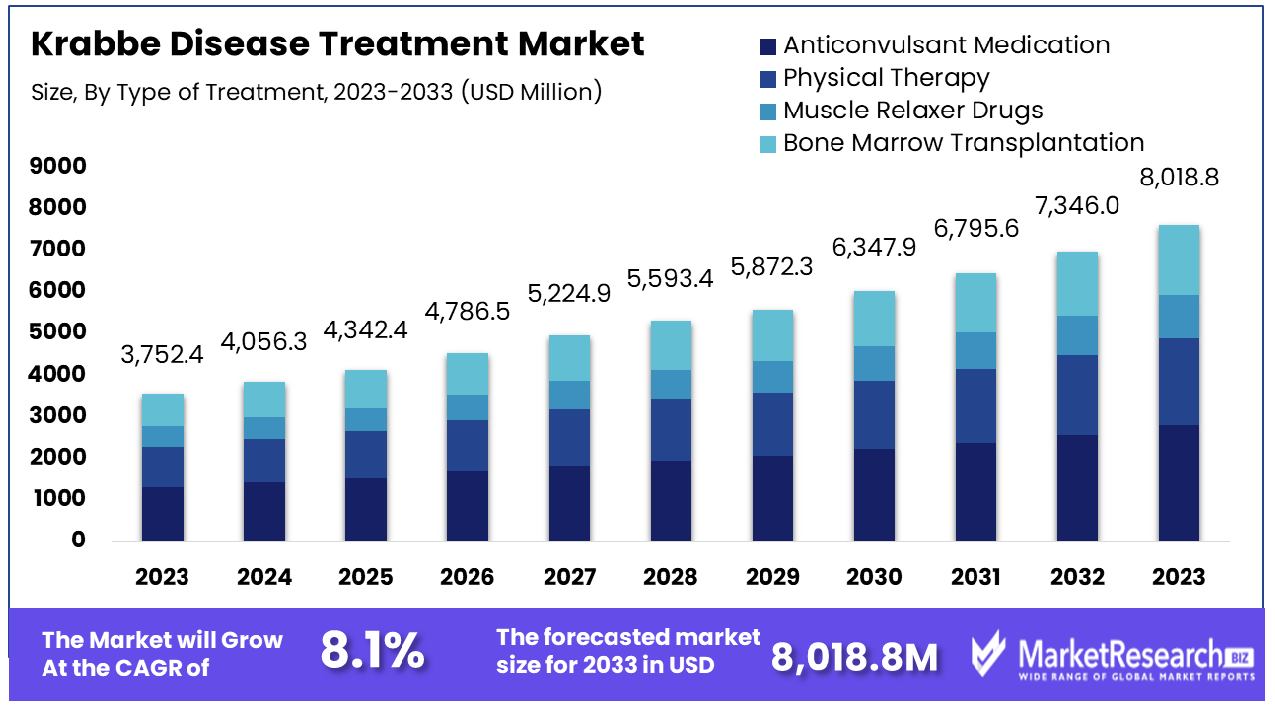
The Krabbe disease treatment market was valued at USD 3,752.4 million in 2023. It is expected to reach USD 8,018.8 million by 2033, with a CAGR of 8.1% during the forecast period from 2024 to 2033.
Rising demand for innovative drugs and the emergence of globoid cell leukodystrophy are among the key drivers behind market expansion. Krabbe disease, also referred to as Krabbe's Disease or Sphingo Leukodystrophy is an inherited, progressive globoid cell leukodystrophy that results in leukodystrophy of globoid cell leukodystrophy characterized by galactocerebroside deficiency.
Also called the galactocerebroside (GALC) condition. Neurological conditions like leukodystrophy and storage disorder (LSD) involve the destruction of protective barriers protecting nerve cells. This shield provides essential protection from injury as well as speedy transference of signals through the body.
In Krabbe disease, the shortage of enzymes in the lysosome results in a harmful buildup that leads to the elimination of myelin. Any kind of damage to the myelin protection shield will cause impulses to gradually slow down and stop, causing the underlying nerve fibers to die eventually.
In etiology, Krabbe disease is caused by the loss of body functions that generate gene mutations coding for GALC. According to the National Centre of Biotechnology Information in August 2023, more than 200 GALC gene mutations were noticed. It is tough to create the phenotype sternness with the genotype, but the large number of deletions, like 30 kilobase gene deletions, is general and anticipates 40% to 50% gene mutation in the infantile method in northern Europe and 35% in Mexican patients. Approx. 50% of the patients with teenager and adult types are heterozygous for a 30kb gene deletion that leads to a reduction in GALC enzyme productivity.
Additionally, in epidemiology, Krabbe’s disease is a type of genetic disorder and its regularity changes with the growing population. It is calculated that 1 per 100,000 live births are taking place in Europe. Among those, 1 per 250,000 was created after analyzing death certificates, but these numbers will increase in Europe. It has a high rate in the Israel Druze community, where 6 per 1000 births are due to consanguineous marriages. Under 4 subtypes, 85% to 90% of the cases are reported of infantile subtypes, which are very severe, and it is rising eventually. The mortality rate is quite high, as 90% are in the first two years of life.
To avoid Krabbe disease, many states in the US are following one standard medical procedure by doing a blood test, and another way of treatment is conducting an MRI that helps to display the differences in a child’s brain that point towards Krabbe disease. Due to a surge in severe neurological cases and treatments, Krabbe disease treatment will experience rapid market expansion over the coming years.
Krabbe Disease Treatment Market Dynamics
Awareness Catalyzes Krabbe Disease Treatment Demand
Healthcare professionals, patient advocacy groups, and government programs who collaborated in efforts to educate healthcare providers and the general public regarding Krabbe disease symptoms, diagnosis, and treatments have led to earlier detection and treatment. Increased awareness has proven essential in improving patients' outcomes as well as demand for efficient therapies; as awareness campaigns reach wider audiences markets for treatments for this disease are expected to expand with rising demand for treatment solutions.
Disease Prevalence Intensifies Treatment Needs
As more cases of Krabbe disease are discovered through diagnosis, the demand for effective treatments increases exponentially. With more patients diagnosed, researchers and pharmaceutical companies must find better ways to treat Krabbe disease; with its increasing incidence expected to drive demand further uphill as markets and markets accelerate growth rate further.
Genetic Testing Advances Drive Market Evolution
Advancements in genetic tests and diagnosis are having an enormously positive effect on the Krabbe market for the treatment of disease. Early and accurate identification through genetic screening can facilitate timely interventions essential to managing illness, while as more genetic tests become widely available and commonplace, the opportunity to detect Krabbe disease earlier increases rapidly, driving demand for treatments. New diagnostic tools improve not only accuracy but efficiency as well - expanding market demand significantly.
Shortage of Qualified Healthcare Providers Restrains Krabbe Disease Treatment Market Growth
Krabbe disease requires special expertise for diagnosis, treatment, and administration of advanced therapies such as hematopoietic stem-cell transplantation. Unfortunately, due to its rarity, many healthcare professionals do not possess sufficient expertise in its management; as a result of this fact there can be a concentration of experts in certain regions or centers; making accessing expert treatments more challenging in underserved and rural regions while restricting market expansion with regards to providing a wider range of solutions.
Limited Pharmaceutical Supplies Hinder Krabbe Disease Treatment Market Expansion
As an intricate disease, Krabbe disease requires specific medications that can be costly and hard to access due to limited markets for these treatments. Their small production runs may lead to shortages; while the process for developing and approving effective new treatments for rare illnesses can also create supply issues; all this leads to limited treatment options readily available limiting patients access and the market expansion for Krabbe disease treatment key companies.
Krabbe Disease Treatment Market Segmentation Analysis
By Type of Treatment Analysis
In the market for Krabbe disease treatment, Anticonvulsant Medication represents the dominant segment. Anticonvulsant medications have become a mainstay of treatment plans for Krabbe disease due to their effectiveness and ease of access, as they are frequently required to manage symptoms like seizures. Anticonvulsants help manage neurological symptoms and enhance living quality for those affected. Their efficiency and access are the primary drivers behind their prominence in treatment plans.
Anticonvulsant medications are an essential element in managing symptoms; other treatment options contribute as well. Physical Therapy may help improve mobility and decrease the spasticity of muscles, whereas Muscle Relaxer Drugs treat muscle-related symptoms. Bone Marrow Transplantation offers another aggressive option that could potentially slow the progression of the disease when done early enough in its course.
By End Users Analysis
Hospitals and Clinics are the primary users in the Krabbe disease treatment market. Their dominance can be attributed to their complex nature, which demands multidisciplinary approaches to management and treatment. Hospitals and Clinics provide essential infrastructure, specialized staff expertise, and access to multiple treatment modalities - making them key centers for patient care. Integrating different services from diagnosis through to ongoing management is integral in providing holistic care to those living with Krabbe disease.
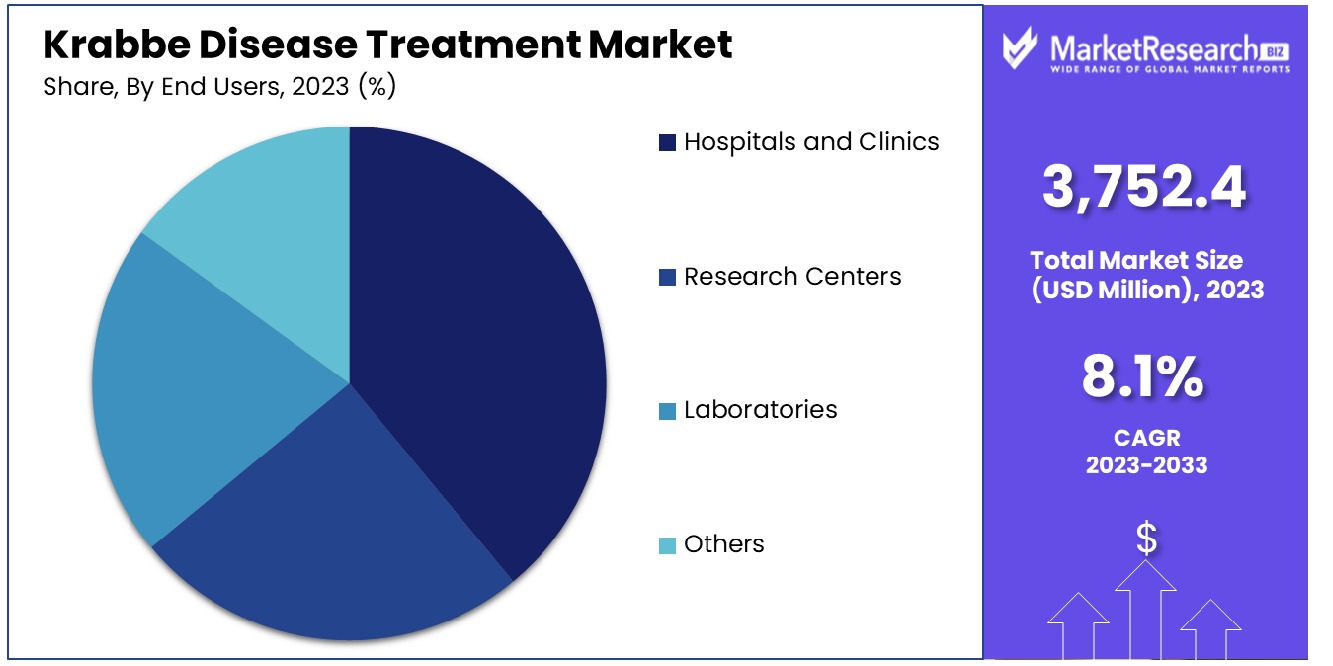
Other end users, including Research Centers and Laboratories, play an essential part in this market. These institutions are at the forefront of creating innovative treatments and contributing to scientific knowledge of Krabbe disease. Their work is essential in expanding treatment options and improving patient outcomes. Additional end users such as specialized care facilities and home care services also play key roles by providing support and care services for Krabbe disease patients; all providing vital components to creating a holistic ecosystem of treatment for Krabbe disease patients.
Krabbe Disease Treatment Industry Segments
By Type of Treatment
- Anticonvulsant Medication
- Physical Therapy
- Muscle Relaxer Drugs
- Bone Marrow Transplantation
By End Users
- Hospitals and Clinics
- Research Centers
- Laboratories
- Others
Krabbe Disease Treatment Market Growth Opportunity
Increasing Clinical Development and R&D Expenditure Boosts Krabbe Disease Treatment Market
As medical knowledge on Krabbe disease advances, so too has its scientific understanding increased, prompting an increased focus on developing effective therapies and treatments. With increased investments in R&D come more clinical trials, advanced genetic research initiatives, and the exploration of new treatment methodologies; all contributing to an expanding market driven by both demand for effective Krabbe disease therapies and increased healthcare industry research initiatives targeting rare genetic disorders.
Rising Need for Streamlined Operations Propels Krabbe Disease Treatment Market
The rising need for streamlined operations in healthcare facilities, particularly those dealing with rare diseases like Krabbe, is creating growth opportunities within the treatment market. Efficient, streamlined operations are crucial in providing timely and effective treatment to patients with rare conditions. Improving operational efficiency can lead to faster diagnosis, better patient management, and more effective treatment implementation. This need drives the demand for advanced diagnostic tools, integrated care approaches, and effective therapeutic solutions.
Krabbe Disease Treatment Market Regional Analysis
North America Dominates with 77.80% Market Share in the Krabbe Disease Treatment Market
North America holds an estimated 77% market share for treatments for Krabbe disease treatments due to advanced healthcare infrastructure, higher awareness levels, and significant investments into rare research into rare disorders. The United States boasts a robust system for orphan drug development which provides incentives to pharmaceutical companies developing therapies for rare conditions like Krabbe disease. Furthermore, its high healthcare costs combined with policies supporting medical research along with genetic tests enable early diagnosis and access to treatments which ultimately accounts for its dominance of this market.
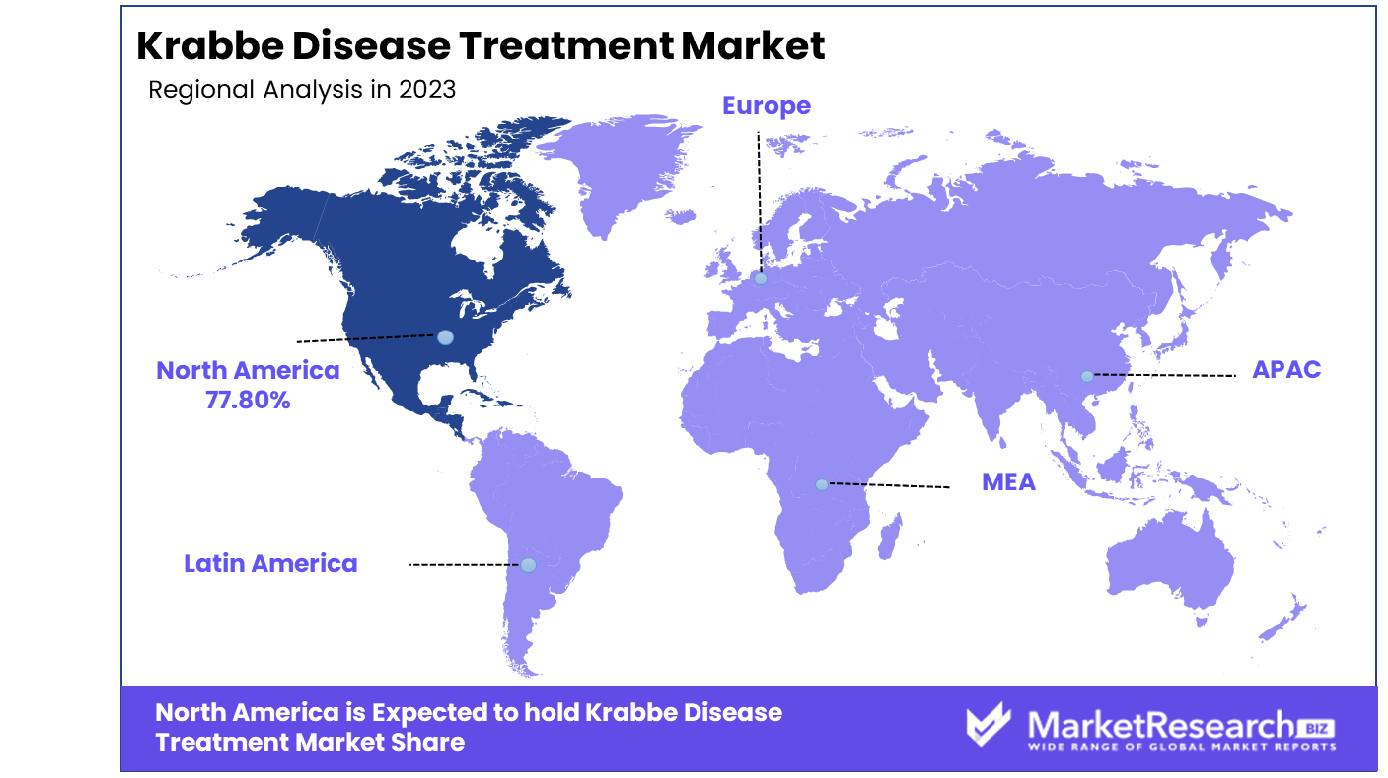
North American markets are characterized by active patient support groups and active advocacy for funding rare diseases, increasing awareness, and further research. Furthermore, collaborations among biotech companies, research institutions, healthcare providers, and biopharmaceutical firms focused on treating Krabbe disease have resulted in innovative treatment solutions being developed; their presence has had a great effect on the market expansion.
Europe: Strong Regulatory Support and Research Initiatives
Europe's Krabbe disease treatment market is driven by strong regulatory support for rare diseases and substantial research initiatives. The European Medicines Agency's policies on orphan drugs incentivize treatment development, while public health systems provide a framework for access to care. Ongoing research collaborations across European countries contribute to advancements in understanding and treating Krabbe disease.
Asia-Pacific: Growing Awareness and Improving Healthcare Infrastructure
Asia-Pacific's Krabbe disease treatment market is emerging, driven by rising awareness of genetic disorders and an improved healthcare infrastructure. Countries like Japan and South Korea are investing heavily in healthcare research on rare diseases; also increasing focus on healthcare service quality improvement and accessing advanced treatments presents potential for market expansion; Asia-Pacific is likely to experience greater demand for Krabbe disease treatments over time as awareness and healthcare systems develop further.
Krabbe Disease Treatment Industry By Region
North America
- The US
- Canada
- Rest of North America
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherlands
- Rest of Europe
Asia-Pacific
- China
- Japan
- South Korea
- India
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of Asia Pacific
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of Middle East & Africa
Krabbe Disease Treatment Market Share Analysis
Krabbe Disease Treatment Market, an emerging yet essential sector for neurological conditions such as Krabbe, is comprised of companies that play a crucial role in shaping therapeutic and diagnostic approaches to these rare disorders. Abbott Laboratories and CENTOGENE N.V. are significant for their contributions to diagnostic advancements, improving early detection and treatment initiation, crucial for patient outcomes in Krabbe disease.
GlaxoSmithKline plc, Johnson & Johnson Services, Inc, and Novartis AG are key players with a broad range of pharmaceutical products, including those that potentially address symptoms and complications of Krabbe disease. Their market influence is marked by extensive research and development capabilities, driving innovation in treatment options.
Pfizer Inc. and Sanofi-Aventis SA contribute with their expertise in developing therapies for rare diseases, reflecting the industry's focus on underserved medical conditions. Shire (now part of Takeda) has long been recognized for its expertise in treating rare genetic disorders, notably Krabbe disease. They were an instrumental force in finding treatment options for this debilitating illness.
Krabbe Disease Treatment Industry Key Players
- Abbott Laboratories
- CENTOGENE N.V.
- GlaxoSmithKline plc
- Johnson & Johnson Services, Inc
- Novartis AG
- Pfizer Inc.
- Sanofi-Aventis SA
- Shire
- Teva Pharmaceutical Industries Ltd.
- UCB Pharmaceuticals
- Acorda Therapeutics Inc.
- Abbvie Inc.
Krabbe Disease Treatment Market Recent Development
- In March 2023, Vertex Pharmaceuticals (MA, USA) licensed ImmunoGen’s antibody-drug conjugate (ADC) technology for gene editing research. ImmunoGen will receive an upfront payment of US$15 million and may receive up to US$337 million in option exercise fees and development and commercial milestone payments per target. This agreement aims to explore ImmunoGen's technology for developing ADCs for transplant conditioning in connection with gene editing.
- In 2022, Forge Biologics: Forge Biologics, a gene therapy contract manufacturing and clinical-stage therapeutics development company based in Columbus, Ohio, operates a 200,000-square-foot facility with 20 cGMP suites. They are a prominent supplier of AAV (adeno-associated virus) vectors and have started offering plasmid manufacturing services.
- In 2021, Emory University partnered with the Georgia Department of Public Health (GA DPH) to begin screening for Krabbe disease as part of a three-year pilot program. This partnership aimed to provide early diagnosis and follow-up care for infants with Krabbe disease, ensuring prompt treatment for affected individuals.
- In April 2021, Forge Biologics, a gene therapy biotech-CDMO hybrid, secured $120 million in funding to expand its capacity for adeno-associated virus (AAV) contract manufacturing services and to triple its workforce.
Report Scope
Report Features Description Market Value (2023) USD 3,752.4 Million Forecast Revenue (2033) USD 8,018.7 Million CAGR (2024-2032) 8.1% Base Year for Estimation 2023 Historic Period 2016-2023 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Type of Treatment(Anticonvulsant Medication, Physical Therapy, Muscle Relaxer Drugs, Bone Marrow Transplantation), By End Users(Hospitals and Clinics, Research Centers, Laboratories, Others) Regional Analysis North America - The US, Canada, Rest of North America, Europe - Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe, Asia-Pacific - China, Japan, South Korea, India, New Zealand, Singapore, Thailand, Vietnam, Rest of Asia Pacific, Latin America - Brazil, Mexico, Rest of Latin America, Middle East & Africa - South Africa, Saudi Arabia, UAE, Rest of Middle East & Africa Competitive Landscape Abbott Laboratories, CENTOGENE N.V., GlaxoSmithKline plc, Johnson & Johnson Services, Inc, Novartis AG, Pfizer Inc., Sanofi-Aventis SA, Shire, Teva Pharmaceutical Industries Ltd., UCB Pharmaceuticals, Acorda Therapeutics Inc., Abbvie Inc. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Abbott Laboratories
- CENTOGENE N.V.
- GlaxoSmithKline plc
- Johnson & Johnson Services, Inc
- Novartis AG
- Pfizer Inc.
- Sanofi-Aventis SA
- Shire
- Teva Pharmaceutical Industries Ltd.
- UCB Pharmaceuticals
- Acorda Therapeutics Inc.
- Abbvie Inc.




