
In Vitro Diagnostics Market by products and services (Reagents & kits, Instruments, Services), by techniques ( Clinical chemistry, Basic metabolic panel, And Other ), by application (Infectious Diseases, Cancer, And Other), by end-user (Hospitals, Laboratories, And Other ) by Region And Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, And Forecast 2023-2032
-
1184
-
Jul 2023
-
186
-
-
This report was compiled by Trishita Deb Trishita Deb is an experienced market research and consulting professional with over 7 years of expertise across healthcare, consumer goods, and materials, contributing to over 400 healthcare-related reports. Correspondence Team Lead- Healthcare Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Quick Navigation
Report Overview
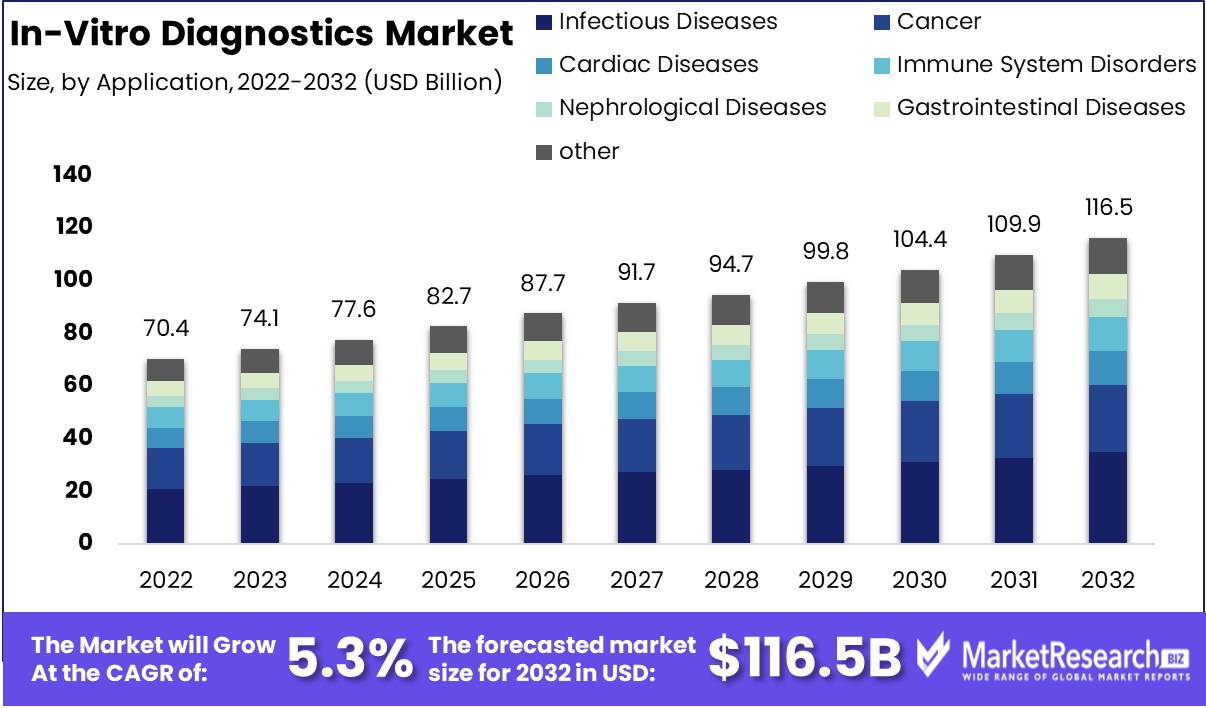
In-Vitro Diagnostics Market size is expected to be worth around USD 116.5 Bn by 2032 from USD 70.4 Bn in 2022, growing at a CAGR of 5.3% during the forecast period from 2023 to 2032.
The In-Vitro Diagnostics a mysterious area with transformative potential, has grown exponentially over the years and shows no signs of slowing down. It empowers healthcare practitioners to make informed treatment and management decisions by providing vital insights into a patient's health status. The In-Vitro Diagnostics Market's captivating innovations, alluring investments, exponential growth, expansive applications, and ethical dilemmas.
A compelling procedure, a diagnostic symphony, unravels diseases and conditions in the In-Vitro Diagnostics Market. This fascinating process, which involves meticulously analyzing biological samples like blood, urine, and tissue, gives healthcare professionals the unmatched ability to diagnose, treat, and manage diseases. In-Vitro Diagnostics is a shining example of contemporary medicine's many victories and amazing inventions.
Driving factors
Escalating prevalence of chronic conditions
The recently published In-Vitro Diagnostics Market research report by Marketresearch.biz sheds light on the positive impact of various market factors. The growth of the in-vitro diagnostics market is being driven by a variety of factors, including the prevalence of chronic diseases, the aging population, the demand for personalized medicine, and the number of cancer cases worldwide.
The increasing prevalence of chronic diseases such as diabetes, cancer, and cardiovascular disease is increasing the demand for precise and effective diagnostic instruments. IVD plays an important role in the early detection and prevention of chronic diseases. The growing elderly population is also a factor in market expansion, as the elderly require consistent medical care, such as constant monitoring and diagnosis. In addition, advances in personalized medicine are driving demand for patient-specific diagnostic and treatment plans.
The rising rate of cancer diagnoses all around the world
There has been a discernible rise in the total number of cancer cases around the globe, which is expected to be the primary factor driving market expansion. In addition, there has been a rising demand for accurate diagnostic tools that may aid in the treatment of cancer. It should be noted that governments all over the world are currently investing in healthcare infrastructure, which should result in the creation of new market prospects for in-vitro diagnostic tools.
Restraining Factors
Compliance with stringent regulatory policies and practices
Regulatory bodies play a crucial role in preserving the quality and safety of medical devices. However, stringent policies and practices can prolong the approval process and increase the total cost of introducing new products to the market. Inadequate regulatory infrastructure and a lack of transparency can hinder the in vitro diagnostics market's expansion. However, there are numerous methods to overcome these obstacles. For example, businesses can invest in research and development to create innovative products that meet regulatory requirements. Additionally, they can utilize technology to streamline the regulatory procedure, thereby reducing costs and time-to-market.
Costly instruments for in vitro diagnostics in developing nations
While the in vitro diagnostics market is expanding swiftly in developed nations, these instruments and tests remain prohibitively expensive in developing nations. This restricts the use of these diagnostic instruments, preventing individuals from receiving timely and accurate diagnoses. However, the situation can be improved by providing financial assistance to hospitals and clinics in emerging economies, facilitating the acquisition of affordable equipment, and establishing more public-private partnerships to improve access to medical care. In addition, businesses can concentrate on delivering sustainable solutions that cater to the specific requirements of developing nations. The Infectious Disease In Vitro Diagnostics market is witnessing rapid growth, driven by the increasing demand for accurate diagnostic tests.
Techniques Analysis
In recent years, the In-Vitro Diagnostics Market has expanded significantly due to technological advancements and a greater emphasis on early disease detection. Among the numerous market segments, the Clinical Chemistry segment has become the most dominant. The segment focuses on the application of chemical and biochemical techniques to analyze biological fluids and diagnose diseases. The segment has numerous applications, such as oncology, diabetes, cardiovascular diseases, and infectious diseases.
The expansion of the Clinical Chemistry segment can be attributed to the prevalence of chronic diseases, the aging population, and the demand for earlier disease diagnosis. Clinical chemistry testing is also commonly used for drug therapy monitoring and therapeutic drug monitoring. The demand from hospital laboratories, reference laboratories, and diagnostic clinics has increased in this market segment.
Emerging economies, such as India, China, and Brazil, are experiencing rapid economic growth, accompanied by a growing emphasis on healthcare infrastructural development. This has been promoting Clinical Chemistry segment adoption.
These nations have made substantial investments in the improvement of hospital infrastructure, patient care, and medical research, which has led to an increase in the demand for diagnostic tests. Governments are also implementing numerous initiatives to enhance the quality of public healthcare, which will increase the utilization of diagnostic tests.The Clinical Chemistry segment has garnered widespread consumer acceptance as a result of its accurate and trustworthy results.
Application Analysis
The Infectious Diseases segment is an additional crucial segment of the In-Vitro Diagnostics Market. This segment examines biological samples for infectious agents such as bacteria, viruses, and fungi. Infectious diseases pose a serious threat to human health, and early detection is essential for effective treatment. Infectious diseases, including HIV, hepatitis, and tuberculosis, are becoming more prevalent, which has contributed to the expansion of the Infectious Diseases segment. In addition, the segment is anticipated to benefit from the growing demand for personalized medication, which will necessitate accurate and precise diagnosis.
Inadequate sanitation, hygiene, and healthcare facilities are contributing to an increase in the prevalence of infectious diseases in emerging economies. The growth of the Infectious Diseases segment will be driven by the various measures taken by the governments of these nations to combat the spread of infectious diseases.
End-user Evaluation
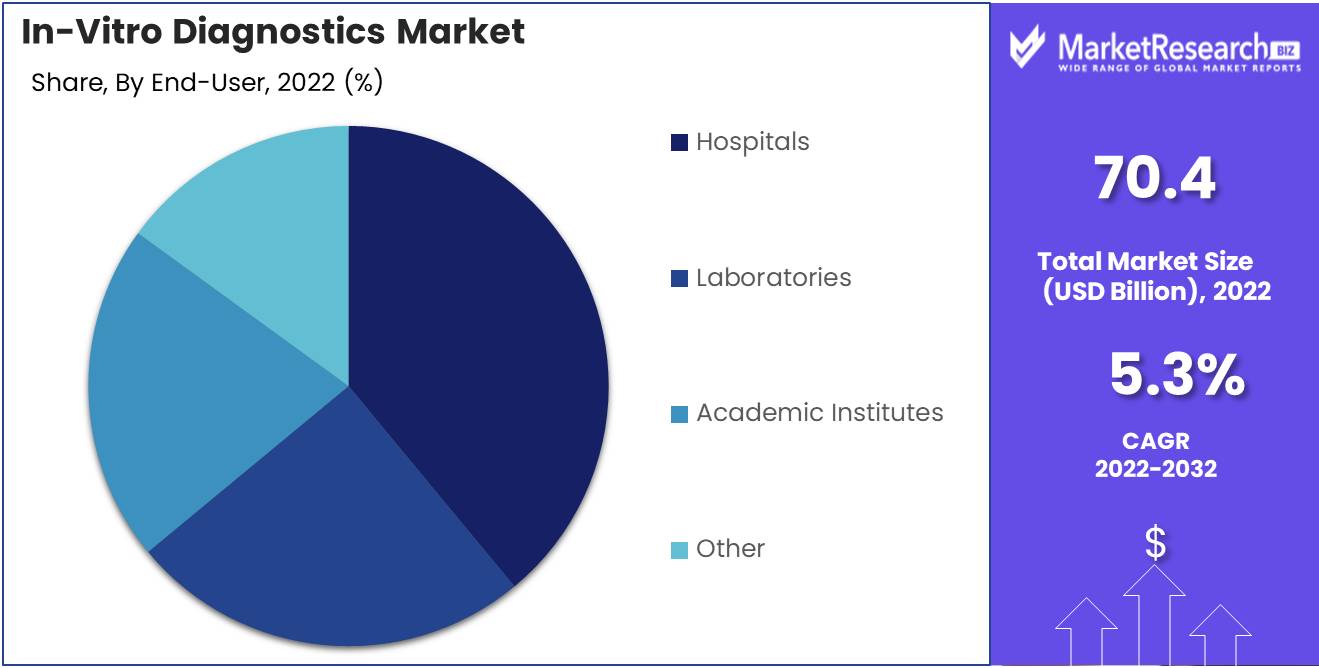
In Vitro Diagnostics Market is dominated by the Hospitals segment. Hospitals are the greatest end-users of diagnostic tests, representing a substantial portion of the market. Hospitals utilize diagnostic tests for a variety of purposes, including disease diagnosis, drug therapy monitoring, predictive and personalized medications, and research. The demand for effective disease diagnosis and treatment has contributed to the expansion of the Hospitals segment. The demand for diagnostic tests is rising as hospitals are seeing an increase in patient volume. Increasing investments in healthcare infrastructure and a greater emphasis on preventive healthcare also contribute to the expansion of the Hospitals segment.
Emerging economies are experiencing accelerated economic growth, which is fueling the expansion of the healthcare industry. The governments of these nations are implementing a variety of initiatives to develop healthcare infrastructure, expand access to healthcare services, and promote public health. The increasing investments in hospital infrastructure will increase the demand for diagnostic tests, which will be advantageous for the Hospitals segment.
Key Market Segments
By products and services
- Reagents & kits
- Instruments
- Services
By techniques
- Clinical chemistry
- Basic metabolic panel
- Electrolyte panel
- Liver panel
- Lipid profile
- Renal profile
- Thyroid function panel
- Specialty chemical tests
- Immunochemistry/Immunoassay
- Enzyme-linked immunosorbent assay (ELISA)
- Radioimmunoassays (RIA)
- Rapid tests
- Western blot
- ELISPOT
- Hematology
- Coagulation & hemostasis
- Clinical microbiology
- Molecular diagnostics (MDx)
- Polymerase chain reaction (PCR)
- Isothermal nucleic acid amplification technology (INAAT)
- Microarrays
- Hybridization
- DNA sequencing & next-generation sequencing
- Other MDX techniques
- Other techniques
By application
- Infectious Diseases
- Cancer
- Cardiac Diseases
- Immune System Disorders
- Nephrological Diseases
- Gastrointestinal Diseases
- Other applications
By end-user:
- Hospitals
- Laboratories
- Academic Institutes
- Other end users (Point-Of-Care testing and Patient self-testing)
Growth Opportunity
Aging Population
A significant element that is contributing to the expansion of the in-vitro diagnostics market is the maturing of the world's population. A population that is getting older increases the risk of chronic diseases and conditions that are associated to diseases, such as cancer and cardiovascular diseases. As a consequence of this, it is anticipated that there will be a rise in demand for in-vitro diagnostic tests in the years to come due to the growing elderly population.
Use of Molecular Diagnostics Becoming More Common
The field of molecular diagnostics is one of the subsets of the IVD market that is expanding at the fastest rate. One of the primary forces behind the expansion of the in-vitro diagnostics market is the rising use of molecular diagnostics in clinical settings, particularly for the testing and diagnosis of infectious diseases and cancer. Molecular diagnostic tools make it possible to diagnose diseases in their earliest stages, which paves the way for earlier treatment and improved clinical results.
Latest Trends
Increasing Number of Requests for Testing of Infectious Diseases
The COVID-19 pandemic has given attention to infectious diseases, which has led to an increase in the demand for diagnostic tests of the highest possible quality. The in vitro diagnostics (IVD) market has reacted by producing a diverse range of products, such as antigen tests, antibody tests, and nucleic acid tests. These tests have taken on an increasingly important role in determining who among a population is infected with the virus, how far it has traveled, and how well vaccines are working.
Demand for items used in IVD is expected to continue to increase as a result of the development of new technologies that enable the rapid identification of infectious diseases. For instance, diagnostic tests based on CRISPR allow for the rapid and precise diagnosis of COVID-19 as well as other viruses. These tests are currently in the process of being developed and may soon be accessible to a wide audience.
Move in the Direction of Decentralized Healthcare
In recent years, there has been a global trend toward decentralized healthcare, with patients obtaining increasing care outside of hospitals and clinics. This trend is expected to continue in the foreseeable future. This trend is increasing demand for IVD devices that are portable, simple to operate, and can be utilized in non-traditional locations such as community health centers, pharmacies, and even in the comfort of one's own home.
In response, IVD manufacturers are working to produce devices that are more compact and user-friendly, while yet providing accurate and dependable findings. Patients can now check their blood sugar levels at home thanks, for instance, to the miniaturization and simplification of glucose monitors specifically designed for diabetics. In a similar vein, point-of-care testing devices for infectious diseases are becoming more widespread. These devices enable medical professionals to quickly diagnose and treat patients in locations that are either underserved or remote.
Growing Problems Caused by Chronic Illnesses Across the Globe
The global population is experiencing an increase in the prevalence of chronic diseases such as diabetes, cardiovascular disease, and cancer. The demand for IVD solutions that can assist identify these diseases and track their progression is being driven higher as a result of this trend. IVD businesses are working to create cutting-edge new diagnostic tests that can detect these diseases at an earlier stage, so enabling patients to begin treatment sooner and potentially improving their results.
For instance, liquid biopsy tests can diagnose cancer with just a blood sample, allowing for earlier identification and action in the event that it is necessary. In addition, biomarker testing for cardiovascular disease are becoming increasingly widespread. These tests enable medical professionals to assess a patient's likelihood of developing heart disease and take preventative measures before the condition worsens.
Regional Analysis
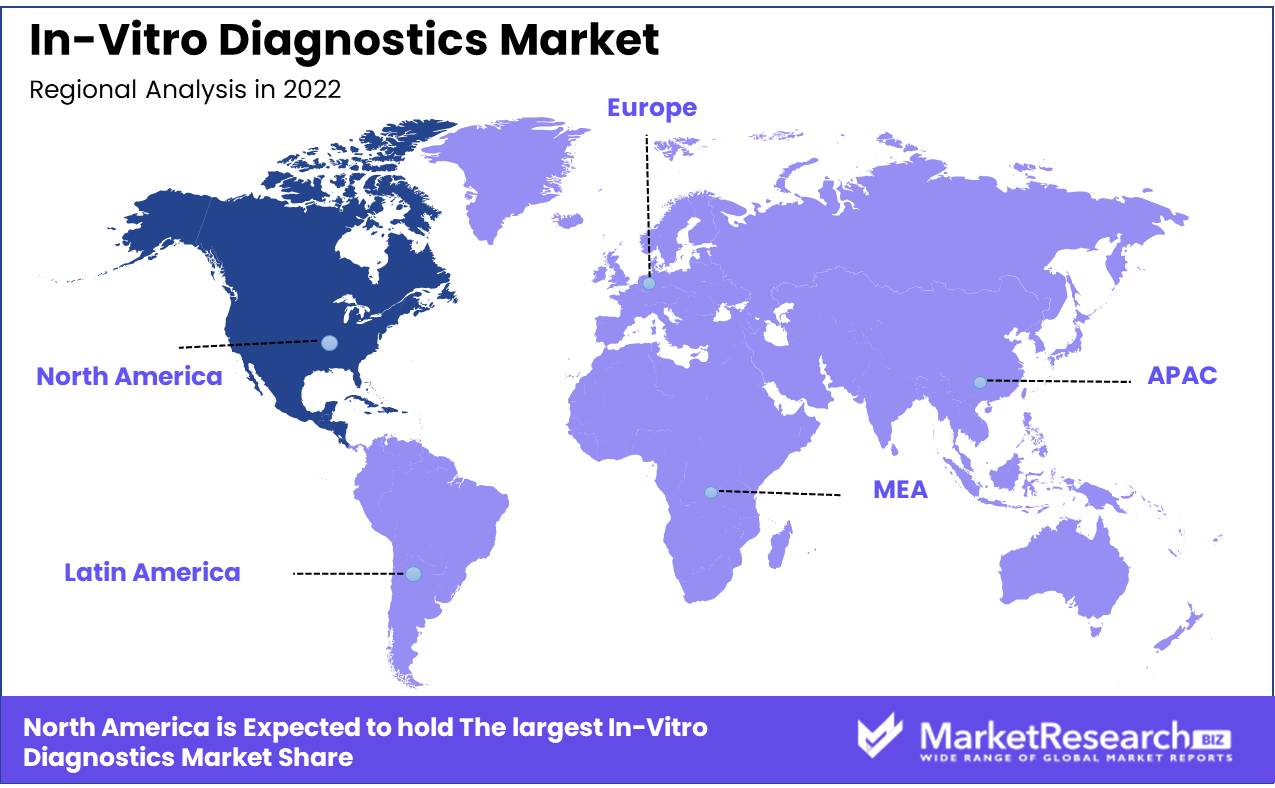
Diabetes and cancer are rising globally at an unprecedented rate. Non-communicable illnesses impact all age categories and demographics, including North Americans. Fortunately, the North American in vitro diagnostics (IVD) industry is rising due to these chronic disorders. In vitro diagnostics tests detect, diagnose, and monitor medical diseases. These tests are done in a lab using various diagnostic equipment.
IVD testing can detect disorders, detect changes in a patient's status, and track therapy efficacy. North America, which includes the US and Canada, is a major in vitro diagnostics market. North America is dominating the IVD market growth with increased awareness of early detection, healthcare spending, and sophisticated healthcare infrastructure.
Chronic disorders including diabetes and cancer are driving the North American IVD market. Diabetes, which affects 34.2 million Americans, is prevalent in the region. High-quality diagnostic tests to monitor and control the condition have driven IVD market growth. Preventive healthcare is also driving the IVD market growth in North America. Screening and diagnostic procedures that can detect diseases early are growing more popular in the region as people become more health-conscious.
Key Regions and Countries
North America
- US
- Canada
- Mexico
Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
New and established firms are joining the In-Vitro Diagnostics (IVD) market. IVD market leaders dominate the global market. The market is highly fragmented with few giant international corporations dominating and other small and medium players capturing the regional market. Roche, Abbott, Danaher, Siemens Healthineers, and Thermo Fisher Scientific are IVD market leaders. These multinational IVD firms offer clinical chemistry, immunoassays, molecular diagnostics, and hematological products.
Roche's IVD market leadership is driven by innovative product development. Abbott's consumer product focus and varied portfolio have helped them succeed. Danaher's acquisition of Beckman Coulter expanded its IVD offerings and strengthened its market position. Siemens Healthineers is developing Point-of-Care (POC) diagnostics to meet the growing demand for decentralized testing. The IVD market has grown thanks to Thermo Fisher Scientific's R&D and acquisition initiatives. Bio-Rad Laboratories, Becton, Dickinson, Johnson & Johnson, and Sysmex Corporation are among IVD market leaders.
Top Key Players in In-Vitro Diagnostics Market
- Roche Diagnostics
- Abbott Laboratories
- Siemens Healthineers
- Danaher Corporation
- Thermo Fisher Scientific Inc.
- Sysmex Corporation
- Bio-Rad Laboratories Inc.
- BioMérieux
- Becton
- Dickinson and Company
- Johnson and Johnson
- Qiagen N.V.
- Ortho-Clinical Diagnostics Inc.
- Diasorin S.P.A.
Recent Development
- In 2023, Thermo Fisher Scientific (US) has recently introduced the rapid RT-PCR Accula Flu A/Flu B Test, which is designed to assist healthcare providers in detecting and differentiating influenza A and B within 30 minutes.
- In 2023, The introduction of this new test is anticipated to have a substantial impact on the In-Vitro Diagnostics Market, as both healthcare providers and patients seek quicker, more accurate testing.
- In 2023, Thermo Fisher Scientific has a long history of innovation in the In-Vitro Diagnostics Market, and this latest innovation demonstrates their dedication to providing cutting-edge patient care solutions.
Report Scope
Report Features Description Market Value (2022) USD 70.4 Bn Forecast Revenue (2032) USD 116.5 Bn CAGR (2023-2032) 5.3% Base Year for Estimation 2022 Historic Period 2016-2022 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By products and services (Reagents & kits, Instruments, Services), By techniques ( Clinical chemistry, Basic metabolic panel, Electrolyte panel, Liver panel, Lipid profile, Renal profile, Thyroid function panel, Specialty chemical tests, Immunochemistry/Immunoassay, Enzyme-linked immunosorbent assay (ELISA), Radioimmunoassays (RIA), Rapid tests, Western blot, ELISPOT, Hematology, Coagulation & hemostasis, Clinical microbiology, Molecular diagnostics (MDx), Polymerase chain reaction (PCR), Isothermal nucleic acid amplification technology (INAAT), Microarrays, Hybridization, DNA sequencing & next-generation sequencing, Other MDX techniques, Other techniques), By application (Infectious Diseases, Cancer, Cardiac Diseases, Immune System Disorders, Nephrological Diseases, Gastrointestinal Diseases, Other applications), By end-user (Hospitals, Laboratories, Academic Institutes, Other end users (Point-Of-Care testing and Patient self-testing)) Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Roche Diagnostics, Abbott Laboratories, Siemens Healthineers, Danaher Corporation, Thermo Fisher Scientific Inc., Sysmex Corporation, Bio-Rad Laboratories Inc., BioMérieux, Becton, Dickinson and Company, Johnson and Johnson, Qiagen N.V., Ortho-Clinical Diagnostics Inc., Diasorin S.P.A. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Roche Diagnostics
- Abbott Laboratories
- Siemens Healthineers
- Danaher Corporation
- Thermo Fisher Scientific Inc.
- Sysmex Corporation
- Bio-Rad Laboratories Inc.
- BioMérieux
- Becton
- Dickinson and Company
- Johnson and Johnson
- Qiagen N.V.
- Ortho-Clinical Diagnostics Inc.
- Diasorin S.P.A.




