Immuno Oncology Market By Type (Immune Cell Therapy (CAR-T), Monoclonal Antibodies, Checkpoint Inhibitors, Cytokines, Cancer Vaccines, Other Types), By End-User (Hospitals & Clinics, Ambulatory Surgical Centers, Cancer Research Institutes, Other End-Users), By Region And Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, And Forecast 2023-2032
-
40497
-
Aug 2023
-
190
-
-
This report was compiled by Trishita Deb Trishita Deb is an experienced market research and consulting professional with over 7 years of expertise across healthcare, consumer goods, and materials, contributing to over 400 healthcare-related reports. Correspondence Team Lead- Healthcare Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Quick Navigation
Report Overview
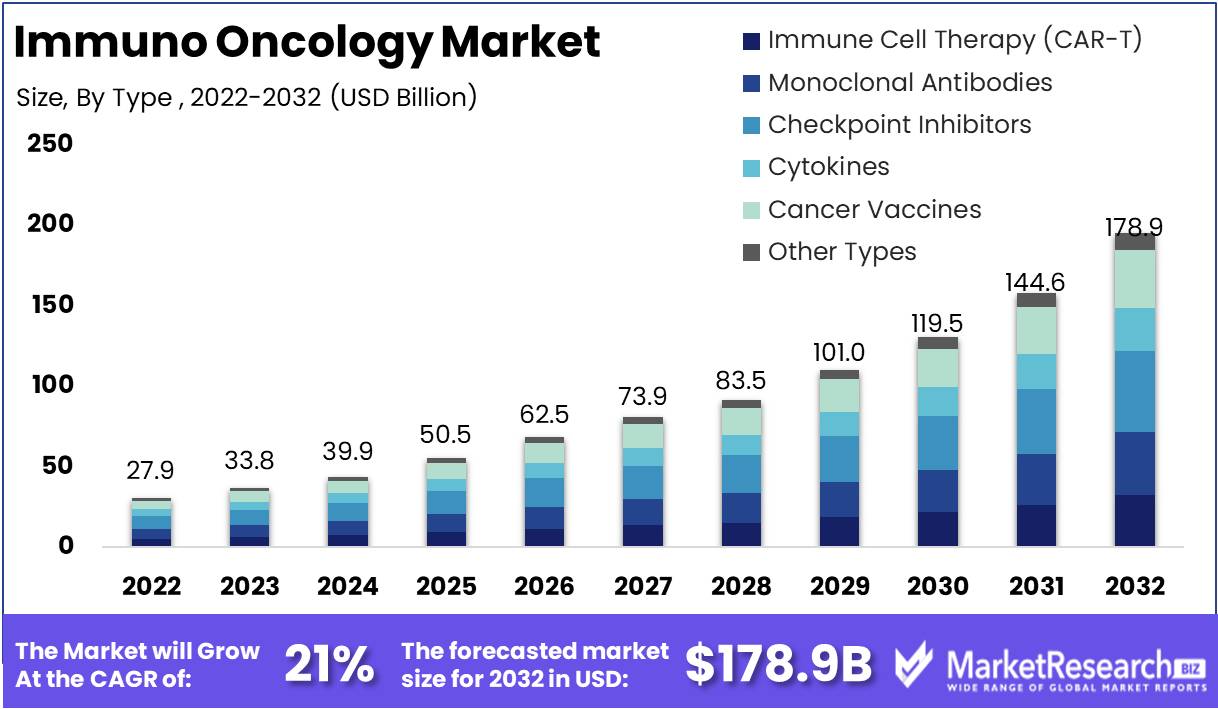
Immuno Oncology Market size is expected to be worth around USD 178.9 Bn by 2032 from USD 27.9 Bn in 2022, growing at a CAGR of 21% during the forecast period from 2023 to 2032.
The immuno oncology market has emerged as a significant field in the pharmaceutical industry, revolutionizing cancer treatment. Immuno oncology, also known as cancer immunotherapy, refers to the utilization of the body's immune system to fight cancer cells. Unlike traditional cancer treatments that directly target cancer cells, immuno oncology focuses on strengthening and enhancing the immune system's ability to recognize and destroy cancer cells. The ultimate goal is to provide patients with more effective, durable, and less toxic treatment options.
The immuno oncology market is of paramount importance due to its potential to transform cancer treatment. Traditional methods such as chemotherapy and radiation therapy often come with severe side effects and limited efficacy. However, immuno oncology offers several advantages. It can effectively target cancer cells while sparing healthy cells, reducing toxicities, and improving patients' quality of life. Additionally, immuno oncology treatments have shown remarkable success in certain cancers, leading to long-term remission and even cure.
The immuno oncology market has seen significant advancements, including immune checkpoint inhibitors that boost the immune system's ability to combat cancer. Pembrolizumab and nivolumab are prominent examples, proving effective against melanoma and lung cancer. Another breakthrough is CAR-T therapy, altering patients' T-cells to target cancer cells. Kymriah and Yescarta are approved CAR-T therapies, showing promising results in treating lymphomas and leukemia with high response rates and extended remission periods.
Despite its potential, the immuno oncology market raises ethical concerns that must be addressed responsibly. Ethical considerations mainly include patient selection, access to treatments, and potential financial burdens on patients and healthcare systems. It is crucial to ensure equitable access to immuno oncology therapies, particularly for patients who may benefit the most. Ethical decision-making frameworks and guidelines are being developed to optimize patient outcomes while addressing these concerns.
The immuno oncology market represents a revolutionary stride in cancer treatment by utilizing the immune system's potential to combat cancer cells effectively. Its importance has grown significantly due to remarkable innovations, substantial investments, and rapid progress. However, ethical considerations and responsible actions are crucial to ensure fair accessibility and optimal patient results. Transparency, explainability, and accountability will remain pivotal in shaping immuno oncology, fostering research, clinical practices, and business approaches. Anticipated advancements and breakthroughs in this maturing market offer hope to patients and revolutionize the oncology domain.
Driving Factors
Advancements in Cancer Immunotherapy and Targeted Therapies
Cancer immunotherapy and targeted therapies have transformed oncology, providing novel treatment options and driving progress in cancer care. The immuno oncology market has surged due to advancements in therapies like immune checkpoint inhibitors and CAR T-cell therapy, bolstering the body's defenses against cancer. Meanwhile, targeted therapies focus on disrupting specific cancer cell pathways through precision medicine, tailoring treatment based on genetic mutations and biomarkers. These innovations have not only improved patient outcomes but also elevated the fight against various types of cancer, increasing survival rates along the way.
Growing Understanding of the Immune System's Role in Cancer Treatment
The interplay between the immune system and cancer has been extensively researched, revealing cancer cells' evasion mechanisms and leading to innovative strategies to bolster the immune system's ability to target and eliminate cancer cells. This understanding has fueled interest and investment in the immuno oncology market, with successful immunotherapies like immune checkpoint inhibitors showing efficacy in various cancers. Moreover, CAR T-cell therapy has revolutionized treatment for blood cancers, genetically modifying immune cells to recognize and combat cancer cells effectively.
Expansion of Personalized Medicine and Precision Oncology
The rise of personalized medicine and precision oncology has revolutionized oncology, leveraging an individual's genetic profile and tumor characteristics to tailor treatment strategies. Advancements in genomic profiling and next-generation sequencing have empowered oncologists to pinpoint genetic alterations and biomarkers, guiding precise treatment decisions. Consequently, the immuno oncology market experiences significant growth as personalized approaches result in better treatment outcomes, reduced toxicities, and enhanced overall survival rates and quality of life for patients.
Increasing Investments in immuno oncology Research
The vast potential of immuno oncology in revolutionizing cancer treatment draws considerable investments in research and development. Pharmaceutical firms, academic institutions, and research organizations are dedicating substantial resources to immuno oncology research, aiming to create novel therapies and enhance existing treatment options. This influx of investments fuels innovation, explores new immunotherapy approaches, deepens cancer biology understanding, and identifies promising therapy targets, ultimately propelling the growth of the immuno oncology market and potentially transforming cancer outcomes.
Restraining Factors
High Cost of immuno oncology Treatments
The immuno oncology market has made remarkable progress, transforming cancer treatment. However, high costs hinder widespread adoption, especially in regions with limited healthcare access. The industry, healthcare professionals, and policymakers aim to enhance affordability. Collaborations between pharmaceutical companies and insurance providers may subsidize treatment expenses, easing the financial strain on patients. Moreover, developing generic or biosimilar versions of immuno oncology drugs could make these treatments more accessible and affordable.
Potential Side Effects and Toxicity Concerns
While immuno oncology therapies hold promise in improving patient outcomes, potential side effects and toxicity concerns exist. Overactive immune responses can lead to immune-related adverse events (irAEs) like inflammation, skin rashes, or gastrointestinal issues. These effects significantly impact patients' quality of life and treatment schedules. However, side effects' severity varies based on cancer type, treatment, and overall health. Continuous monitoring and collaboration between healthcare professionals and patients can manage side effects proactively. Ongoing research aims to refine immuno oncology therapies, enhancing treatment tolerability.
Regulatory and Reimbursement Challenges
Adopting immuno oncology treatments faces obstacles due to regulatory hurdles and reimbursement challenges. Stringent evaluations by regulatory agencies extend the time to market, while conflicting regulations across regions cause complexities and delays. Reimbursement acceptance and affordability depend on robust clinical and cost-effectiveness evidence, leading to time-consuming and resource-intensive clinical trials and evaluations for pharmaceutical companies.
Potential Resistance and Immune Evasion Mechanisms
The immuno oncology market faces challenges due to cancer cells developing resistance and evading the immune system. While some patients respond well to immuno oncology therapies, others don't. Ongoing research aims to understand immune evasion and resistance mechanisms and develop strategies like studying tumor microenvironments, exploring immunomodulatory agents, and investigating combination therapies to enhance the immune response. Innovations like personalized medicine and targeted therapies offer potential in overcoming resistance by tailoring treatments based on individual genetic and immune system characteristics, maximizing immuno oncology's effectiveness.
Type Analysis
In the immuno oncology market, checkpoint inhibitors dominate as a favored immunotherapy, effectively blocking proteins that hamper the immune response against cancer cells. These promising drugs have gained popularity among healthcare providers due to their success in treating various cancers.
Economic development in emerging economies fuels the adoption of checkpoint inhibitors, with increased healthcare spending and a focus on enhancing cancer treatment options. The rising middle class with higher disposable incomes also contributes to the demand for advanced cancer therapies.
The checkpoint inhibitors segment is expected to register the fastest growth rate in the immuno oncology market over the forthcoming years. This can be attributed to several factors, including the growing number of clinical trials and research studies exploring the efficacy of these drugs. Pharmaceutical companies are investing heavily in the development of new checkpoint inhibitors, with many drugs currently in the pipeline.
Indication Analysis
The immuno oncology market has seen a surge in the dominance of benign tumors, non-cancerous growths that don't spread to other areas. Though not life-threatening, they still cause discomfort, increasing the need for better treatments. Economic growth in emerging economies has boosted adoption, along with improved healthcare infrastructure.
Benign tumors are gaining prominence in the immuno oncology market, similar to checkpoint inhibitors. Emerging economies' economic development drives treatment adoption. With a rising prevalence of benign tumors and growing awareness, patients seek early medical intervention, leading to a surge in demand for minimally invasive therapies.
The immuno oncology market witnesses rapid growth in the benign tumors segment. Advanced medical technology and diverse treatment options contribute to this trend. Ongoing research efforts aim to develop innovative and targeted therapies for benign tumors, meeting the increasing demand for effective solutions.
End-User Analysis
In the immuno oncology market, the hospitals and clinics segment has emerged as the dominant end-user. Hospitals and clinics play a crucial role in providing cancer patients with services such as diagnosis, treatment, and follow-up care. These facilities have the infrastructure, medical apparatus, and healthcare professionals necessary to administer immunotherapy treatments effectively.
The economic growth of emergent economies has had a significant impact on the adoption of immuno oncology treatments in hospitals and clinics. As these nations continue to invest in healthcare infrastructure, the quality and accessibility of cancer care services have improved significantly. This has led to an increase in patients seeking treatment at hospitals and clinics.
Hospitals' and clinics' dominance in the immuno oncology market has also been influenced by consumer attitudes and behaviors toward hospitals and clinics. Due to the variety of services provided, access to specialized medical professionals, and assurance of quality care, patients frequently prefer to seek treatment in established healthcare facilities. The high demand for hospitals and clinics as end-users in the field of immuno oncology can be attributed to the trust placed in them.
In the future years, it is anticipated that the hospitals and clinics segment will experience the highest growth rate. This is due to the continuous advancements in medical technology, the establishment of specialized cancer centers within hospitals, and the increasing prominence of immunotherapy as an effective cancer treatment. In addition, the rising burden of cancer on healthcare systems has resulted in the expansion of hospitals and clinics, further propelling the expansion of this market segment.

Key Market Segments
By Type
- Immune Cell Therapy (CAR-T)
- Monoclonal Antibodies
- Checkpoint Inhibitors
- Cytokines
- Cancer Vaccines
- Other Types
By Indication
- Benign Tumors
- Melanoma
- Lung Cancer
- Blood Cancer
- Blader Cancer
- Renal Cell Carcinoma
- Other Indications
By End-User
- Hospitals & Clinics
- Ambulatory Surgical Centers
- Cancer Research Institutes
- Other End-Users
Growth Opportunity
Harnessing the Power of Genomic Medicine
Embracing genomic medicine is a promising growth avenue in immuno oncology. Leveraging sequencing advancements and genetic analysis provides valuable insights into tumor characteristics, leading to personalized therapies targeting specific genetic features. Integrating genomics in research and clinical practices through collaboration among stakeholders can revolutionize cancer treatment and drive unprecedented growth in the immuno oncology market.
Exploring Combination Therapies Beyond Immunotherapeutics
The immuno oncology market's growth goes beyond standalone immunotherapies, with researchers exploring combinations with targeted therapies, chemotherapy, and radiation. Collaboration among institutions, pharmaceutical companies, and healthcare professionals is vital for optimal combinations and clinical trial design. Embracing combination therapies expands treatment options and fosters innovative approaches to combat cancer, leading to immense market growth.
Leveraging Artificial Intelligence and Machine Learning
Integrating AI and ML in the immuno oncology market optimizes treatment outcomes and patient care by utilizing clinical and genomic data to predict responses to immunotherapies and identify therapeutic targets. Collaboration between industry leaders, academic institutions, and technology providers ensures accurate analysis of complex datasets, enabling informed decisions for treatment selection and monitoring responses. This enhances market efficiency and fosters a personalized and precise approach to cancer care.
Latest Trends
Demand for Personalized and Neoantigen-Targeted Cancer Vaccines
The field of personalized medicine has revolutionized cancer treatment by tailoring therapies to individual patients. One area of focus is the development of personalized cancer vaccines that target neoantigens. Neoantigens are unique antigens produced by cancer cells due to genetic mutations. By identifying and targeting these neoantigens, researchers are exploring vaccines that can elicit a robust immune response against cancer cells, thereby enhancing treatment effectiveness. This personalized approach holds great potential for improving patient outcomes and reducing adverse effects.
Utilization of Immune-Oncology Biomarkers for Treatment Selection
In the era of precision medicine, identifying predictive biomarkers for immunotherapy response has become paramount. Immune-oncology biomarkers, such as programmed death-ligand 1 (PD-L1) expression and tumor mutational burden (TMB), help clinicians determine patient eligibility and predict treatment outcomes. PD-L1 expression acts as a surrogate marker for immune checkpoint inhibitor response, while TMB reflects the overall mutational load of a tumor, influencing its immunogenicity. By leveraging these biomarkers, physicians can make informed decisions on selecting the most appropriate immunotherapies for individual patients.
Rise of Combination Therapies and Immunotherapy-Based Combinations
As the understanding of cancer immunology deepens, researchers are exploring the potential synergy between different immunotherapies and traditional therapies. Combination therapies, such as immune checkpoint inhibitors combined with targeted therapies or chemotherapy, have shown improved response rates and survival outcomes in several cancer types. Additionally, novel immunotherapy-based combinations, such as combining adoptive cell therapies with immune checkpoint inhibitors, are under investigation to unleash the full potential of the immune system against cancer. These innovative approaches hold immense promise for achieving durable responses and long-term remissions.
Regional Analysis
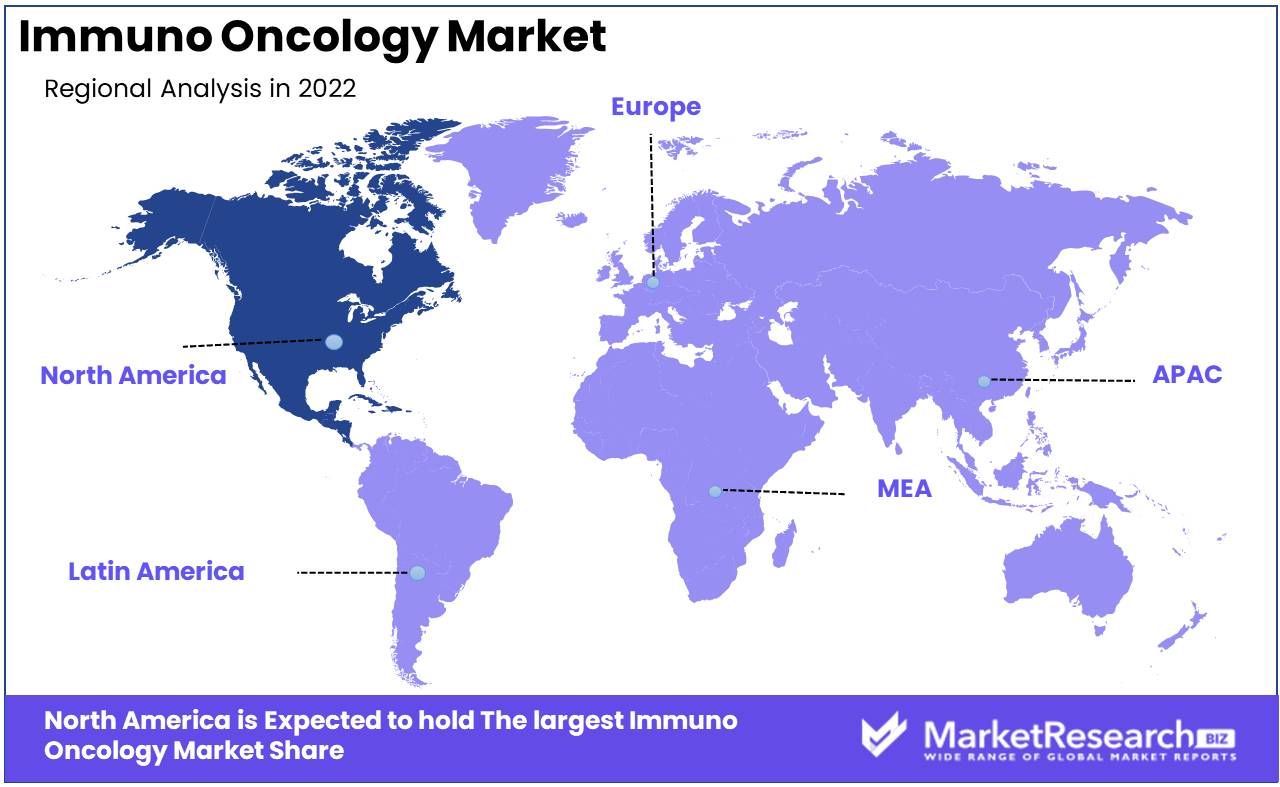
North America Continent As the leader of the global immuno oncology market, the United States and Canada dominate the market for immuno oncology. Significant progress has been made in cancer research and treatment in the region, resulting in an abundance of innovative innovations. immuno oncology has advanced to the vanguard in North America thanks to its well-established healthcare infrastructure, extensive research facilities, and collaborative ecosystem.
Numerous factors are responsible for North America's dominant position in the immuno oncology market. In the first place, the presence of leading pharmaceutical and biotechnology companies in the region has accelerated the development of this discipline. These companies make substantial investments in research and development, allowing them to introduce novel and effective therapies. Their expertise contributes to innovation, attracting global attention and solidifying the region's position as an immuno oncology pioneer.
The dominance of North America in the immuno oncology market is also bolstered by a strong market presence and pervasive adoption of these therapies. The region has a greater patient awareness and acceptance of immuno oncology treatments, resulting in increased market penetration and demand. In addition, North American healthcare providers are more likely to incorporate immuno oncology therapies into their treatment protocols due to their demonstrated efficacy and favorable outcomes.
When analyzing industry collaborations and partnerships, North America's leadership in the immuno oncology market is evident. To spur innovation and accelerate the development of breakthrough therapies, pharmaceutical titans form alliances with academic institutions and entrepreneurs in the region. These collaborations foster the exchange of knowledge and the sharing of resources, thereby bolstering North America's dominance in the immuno oncology industry.
Key Regions and Countries
North America
- US
- Canada
- Mexico
Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
Novartis AG, headquartered in Switzerland, leads in groundbreaking immuno oncology treatments. CAR-T cell therapy, a significant breakthrough, genetically modifies a patient's immune cells to fight cancer. Novartis' Kymriah, the first approved CAR-T therapy, shows remarkable efficacy in leukemia and lymphoma treatment. Research continues to extend its application for other cancers.
Swiss multinational F. Hoffmann-LA Roche pioneers immuno oncology with Tecentriq, empowering the immune system against cancer. Their commitment to personalized medicine is evident through companion diagnostics, identifying patients who benefit from immuno oncology treatments. Roche's research reshapes cancer treatment by combining diagnostics with innovative therapies.
U.K.-based GSK Plc. leads in immuno oncology with dostarlimab, unleashing the immune system against cancer. Targeting PD-1, dostarlimab eradicates cancer cells. GSK Plc. explores novel treatment combinations, aiming to elevate cancer patients' care standards.
Eli Lilly and Company in the U.S. transforms immuno oncology with Cyramza targeting the VEGF receptor pathway. The drug promotes an immune response against cancer. Eli Lilly focuses on identifying biomarkers to personalize treatment strategies and enhance immuno oncology therapy success.
Top Key Players in Immuno Oncology Market
- Bristol Myers Squibb Company (US)
- Novartis AG (Switzerland)
- F. Hoffmann-LA Roche Ltd. (Switzerland)
- Merck & Co. Inc. (US)
- GSK Plc. (UK)
- Eli Lilly and Company (US)
- Fresenius Kabi AG (Germany)
- Pfizer Inc. (US)
- AbbVie Inc. (US)
- Genentech Inc. (US)
- Sanofi (France)
- AstraZeneca (UK)
Recent Development
- In 2023, Global pharmaceutical leader Bristol Myers Squibb announced today the impending launch of their highly anticipated immuno oncology drug, Breo-Zevlin. Slated for release this innovative therapy represents a ray of hope for patients battling lung cancer. With its cutting-edge approach, Breo-Zevlin is expected to revolutionize treatment outcomes, offering potentially life-saving benefits.
- In 2022, Merck & Co. and GlaxoSmithKline are set to join forces in an ambitious partnership aimed at developing a groundbreaking immuno oncology drug, GSK2857916. Announced this collaboration showcases the commitment of these international healthcare powerhouses to advancing cancer treatment. By combining their expertise and resources, Merck & Co. and GlaxoSmithKline are poised to significantly impact the lives of cancer patients worldwide.
- In 2021, Roche, a leading pioneer in the field, made waves as it revealed its strategic acquisition of Spark Therapeutics. This move solidifies Roche's dedication to spearheading advancements in gene therapies for cancer treatment. Spark Therapeutics' cutting-edge technologies and breakthrough discoveries further strengthen Roche's position in the immuno oncology market. Their collective efforts hold immense promise for patients in need of effective gene-based cancer treatments in the near future.
- In 2020, Emphasizing the ongoing commitment to pushing the boundaries of cancer therapeutics, Novartis announced the imminent release of their groundbreaking immuno oncology drug, Kisqali. Set to launch Kisqali raises hopes for patients battling breast cancer. Novartis' relentless pursuit of groundbreaking treatments reflects its dedication to providing innovative solutions and drastically improving patient outcomes.
Report Scope
Report Features Description Market Value (2022) USD 27.9 Bn Forecast Revenue (2032) USD 178.9 Bn CAGR (2023-2032) 21% Base Year for Estimation 2022 Historic Period 2016-2022 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Type (Immune Cell Therapy (CAR-T), Monoclonal Antibodies, Checkpoint Inhibitors, Cytokines, Cancer Vaccines, Other Types)
By Indication (Benign Tumors, Melanoma, Lung Cancer, Blood Cancer, Blader Cancer, Renal Cell Carcinoma, Other Indications)
By End-User (Hospitals & Clinics, Ambulatory Surgical Centers, Cancer Research Institutes, Other End-Users)Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Bristol Myers Squibb Company (US), Novartis AG (Switzerland), F. Hoffmann-LA Roche Ltd. (Switzerland), Merck & Co. Inc. (US), GSK Plc. (UK), Eli Lilly and Company (US), Fresenius Kabi AG (Germany), Pfizer Inc. (US), AbbVie Inc. (US), Genentech Inc. (US), Sanofi (France), AstraZeneca (UK) Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Bristol Myers Squibb Company (US)
- Novartis AG (Switzerland)
- F. Hoffmann-LA Roche Ltd. (Switzerland)
- Merck & Co. Inc. (US)
- GSK Plc. (UK)
- Eli Lilly and Company (US)
- Fresenius Kabi AG (Germany)
- Pfizer Inc. (US)
- AbbVie Inc. (US)
- Genentech Inc. (US)
- Sanofi (France)
- AstraZeneca (UK)




