Gonorrhea Testing Market By Test Type (Nucleic Acid Amplification Tests (NAATs), Culture-Based Tests, Gram Stain, Other), By Sample Type (Blood, Lymph Fluid, Urine, Throat Swab), By End-User (Hospitals and Clinics, Laboratories), By Region And Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, And Forecast 2023-2032
-
37518
-
June 2023
-
137
-
-
This report was compiled by Correspondence Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Quick Navigation
Report Overview
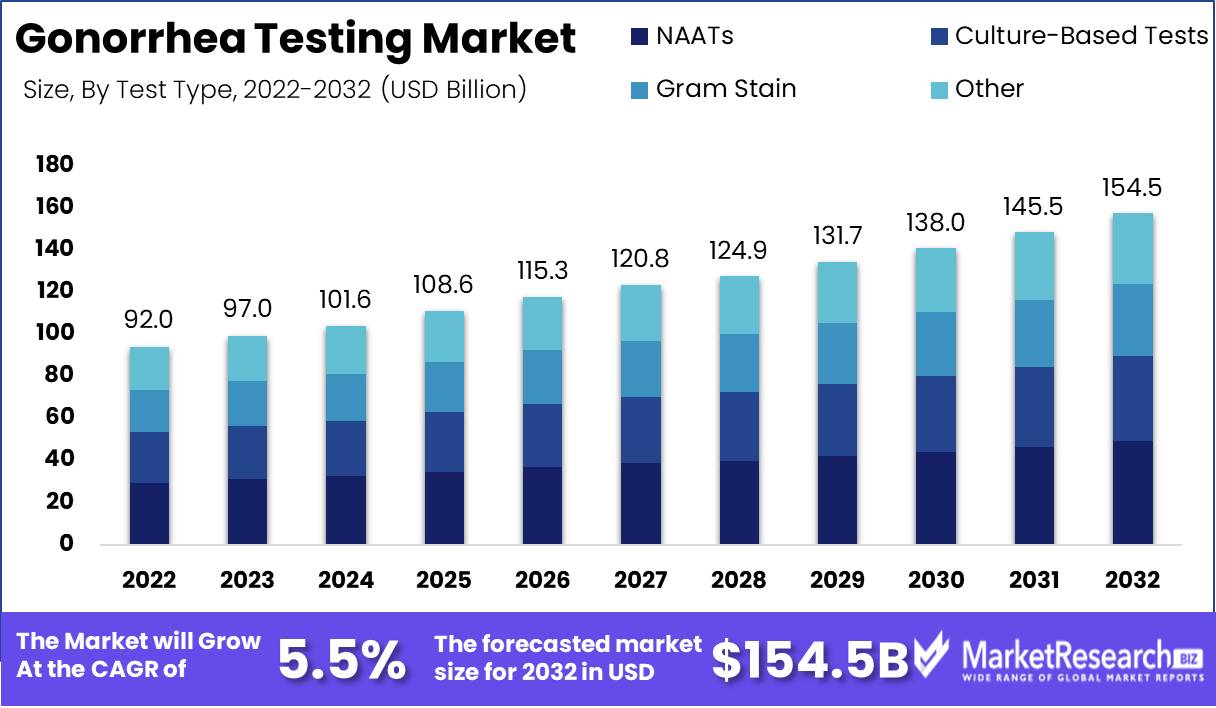
Gonorrhea Testing Market size is expected to be worth around USD 154.5 Bn by 2032 from USD 92 Bn in 2022, growing at a CAGR of 5.5% during the forecast period from 2023 to 2032.
The Global Gonorrhea Testing Market Report is a comprehensive analysis, a sexually transmitted disease. The report offers a comprehensive examination of the market, its growth potential, notable innovations, significant investments, and incorporation into products and services. In addition, the report emphasizes the ethical issues encircling this market, such as transparency, explicability, and accountability. The Global Gonorrhea Testing Market focuses on testing for gonorrhea, a sexually transmitted disease. Gonorrhea is a prevalent STI that, if left untreated, can result in severe health complications. As more people become aware of the hazards of gonorrhea and the necessity of testing for it, the global Gonorrhea Testing Market is anticipated to expand significantly in the coming years.
The objective of the Global Gonorrhea Testing Market is to provide accurate and dependable gonorrhea testing. This testing can aid in preventing the spread of this STI and reducing the number of individuals affected by its complications. There are both traditional laboratory-based testing methods and innovative testing methods on the market. The significance of gonorrhea testing cannot be exaggerated. Gonorrhea is a prevalent STI that, if left untreated, can result in severe health complications, such as infertility and an increased risk of HIV transmission. Detection and treatment at an early stage can prevent these complications and enhance patient outcomes.
In recent years, the Global Gonorrhea Testing Market has witnessed a number of noteworthy advancements. For instance, swift point-of-care diagnostics for gonorrhea now provide results within minutes. In addition, there are more recent testing methods that employ molecular techniques to detect the presence of gonorrhea bacteria.
Numerous healthcare providers and hospitals offer gonorrhea testing to patients, making the healthcare industry a major investor in the Global Gonorrhea Testing Market. In addition, public health agencies and pharmaceutical companies have invested in the market because they believe gonorrhea testing has the potential to reduce the transmission of this STI.
Driving factors
Increasing Gonorrhea Prevalence
During the period of forecast, the Global Gonorrhea Testing Market is anticipated to grow significantly. The increasing prevalence of gonorrhea worldwide is one of the factors fueling this expansion. Demand for early detection and treatment of gonorrhea is on the rise due to advances in diagnostic techniques and rising awareness of sexually transmitted infections (STIs).
Initiatives for Public Health and STI Prevention
In addition, public health initiatives for STI prevention play a significant role in driving market growth. As governments and healthcare organizations prioritize STI prevention and the creation of a secure sexual environment, gonorrhea testing is anticipated to increase in prevalence.
Impact of Regulatory Changes on the Market
In addition to these factors, significant regulatory changes may also have an impact on the Global Gonorrhea Testing Market. For example, the FDA recently approved the use of a rapid diagnostic testing of stds, Gonorrhea and Chlamydia, which has the potential to alter the testing landscape in the United States.
Impacts of Emerging Technologies on the Future
In addition, emerging technologies such as point-of-care diagnostics may have a future impact on the market. Point-of-care tests are transportable, simple to use, and yield immediate results, making them ideal for remote areas.
Emerging Trends and Alterations in Consumer Conduct
Emerging trends and alterations in consumer behavior may have an impact on the market. With the rise of telemedicine and virtual consultations, the demand for remote testing options is growing. Market players are investigating methods to meet this demand and provide consumers with convenient testing options.
Restraining Factors
Stigma associated with STI testing inhibits market expansion
The stigma associated with sexually transmitted infections has a significant negative impact on the gonorrhea testing market. Due to the social stigma associated with sexually transmitted infections (STIs), such as gonorrhea, people frequently feel humiliated or bashful to get tested. This stigma is also fueled by a lack of knowledge and education about STIs, which discourages individuals from seeking testing and treatment.
Difficulties in Reaching Vulnerable Populations
Reaching at-risk populations for gonorrhea testing is a significant market challenge. More susceptible to gonorrhea are individuals with multiple sexual partners, men who have sex with males, and those who inject narcotics intravenously. However, social isolation, lack of education, and mistrust of healthcare systems make it challenging to reach these populations and provide testing services.
Possible Restriction of Diagnostic Tests
The limitations of gonorrhea diagnostic procedures may include false-positive or false-negative results. False negatives can lead to missed diagnoses, whereas false positives can result in unnecessary treatment and stress. Vital to the success of the gonorrhea testing market is ensuring the accuracy and dependability of diagnostic tests.
Financial Limitations on Healthcare Systems
The testing for gonorrhea is impeded by the financial constraints of healthcare systems. Tests, treatments, and follow-up care can be expensive, especially for those with limited financial resources. In addition, the accessibility and availability of testing and treatment centers may be limited, which may further impede the testing process.
Test Type Analysis
Gonorrhea testing is dominated by NAATs. Due to increased gonorrhea rates, sexually transmitted disease awareness, and testing technology developments, the Global Gonorrhea Testing Market is growing. Test, sample, end-user, and region segment the market. Due to its accuracy, speed, and reliability, the Nucleic Acid Amplification Tests (NAATs) Segment dominates the Global Gonorrhea Testing Market. NAATs use nucleic acid sequences to detect gonorrhea. The test amplifies bacterium DNA or RNA, making it simpler to identify low infection levels that standard culture techniques may miss. The gonorrhea test is sensitive and specific.
NAAT demand is rising due to early STD identification and therapy. Health-conscious consumers are preventing STDs. Convenience and privacy boost NAAT adoption. NAATs use a urine or vaginal swab sample and produce findings within hours, unlike culture. This simplifies gonorrhea and STD testing for customers.
Sample Type Analysis
The Urine Segment Dominates the Global Gonorrhea Testing Market. Due to its ease, non-invasiveness, and accuracy, the Urine Segment dominates the Global Gonorrhea Testing Market. Urine sample collection is simple and non-invasive. Consumers prefer urine samples over swab or smear testing. The Global Gonorrhea Testing Market's Urine Segment is gaining popularity due to its ease and non-invasiveness. Non-invasive STI testing are in demand due to early identification and treatment. Urine samples are straightforward to collect and provide accurate and consistent results in a few hours.
The Urine Segment of the Global Gonorrhea Testing Market is expected to expand the quickest. Urine sample collection is consumer-friendly and may be done without medical help, driving expansion. Urine sample collection is non-invasive, which should boost the Urine Segment.
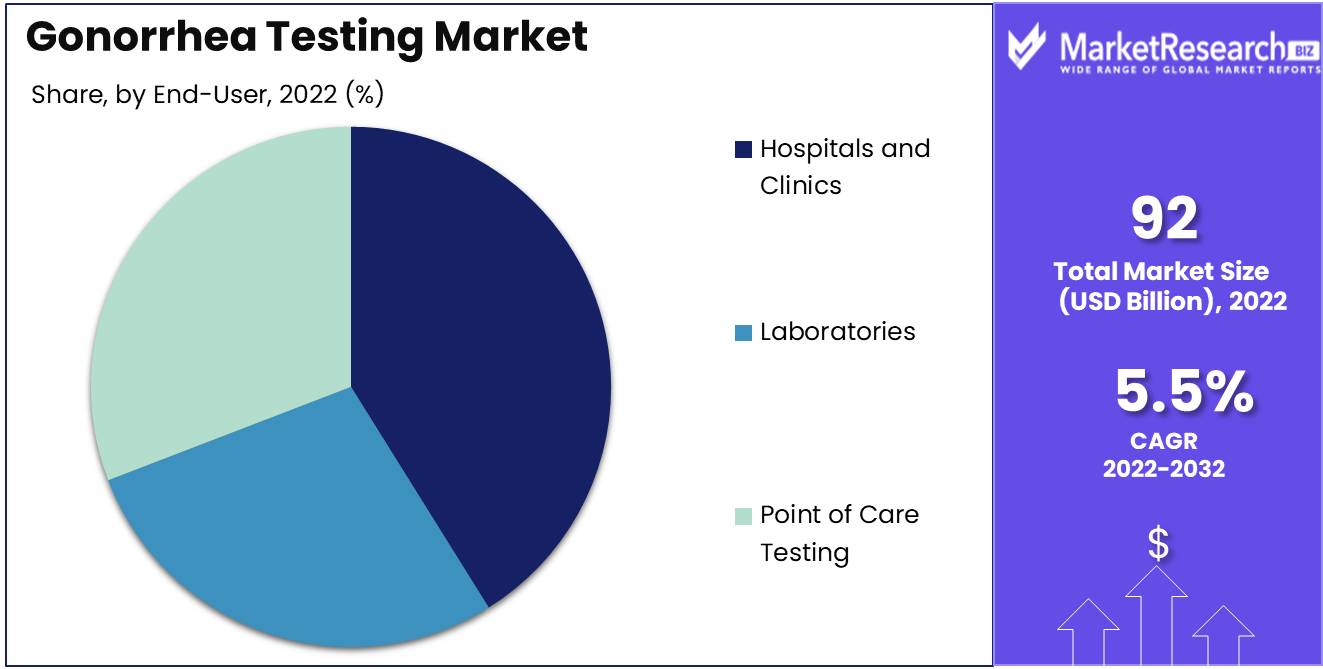
End-User Analysis
The Hospitals and Clinics Segment Dominates the Global Gonorrhea Testing Market. Due to the availability of a greater variety of diagnostic tests, advanced testing techniques, and a greater number of gonorrhea cases, the Hospitals and Clinics Segment dominates the Global Gonorrhea Testing Market. The presence of sophisticated diagnostic technologies and laboratories in hospitals and clinics facilitates the accurate diagnosis of gonorrhea.
Consumers favor hospitals and clinics for sexually transmitted disease (STD) diagnostic testing due to their advanced diagnostic technologies and expertise. Hospitals and clinics offer a greater variety of diagnostic tests and employ highly-trained medical personnel, which increases the accuracy of gonorrhea diagnosis. The trend toward early detection and treatment of sexually transmitted diseases is also driving demand for healthcare services. In the coming years, it is anticipated that the Hospitals and Clinics Segment of the Global Gonorrhea Testing Market will experience the quickest growth rate. The expansion is driven by the availability of sophisticated diagnostic technologies and expertise, which make gonorrhea diagnosis simpler and more accurate.
Key Market Segments
By Test Type
- Nucleic Acid Amplification Tests (NAATs)
- Culture-Based Tests
- Gram Stain
- Other Test Types
By Sample Type
- Blood
- Lymph Fluid
- Urine
- Throat Swab
- Penis/Vaginal Swab
By End-User
- Hospitals and Clinics
- Laboratories
- Point of Care Testing
Growth Opportunity
Development of Rapid and Accurate Gonorrhea Diagnostic Tests
The growth potential of the Global Gonorrhea Testing Market appears optimistic for both healthcare professionals and researchers, with the rapid and accurate development of gonorrhea diagnostic tests playing a crucial role. Newer technologies, such as nucleic acid amplification tests (NAATs) and point-of-care tests (POCTs), provide quicker and more accurate results, thereby increasing the diagnostic and therapeutic efficacy of medical professionals.
Expansion of STI Testing Campaigns and Programs
The expansion of STI testing initiatives and campaigns has contributed significantly to the development of the Global Gonorrhea Testing Market. The significance of regular testing and screening for sexually transmitted infections, such as gonorrhea, is emphasized by public health agencies and advocacy groups worldwide. As a consequence, more people are being tested, which increases the need for enhanced testing methods and technologies.
Point-of-Care Testing Solution Integration
The integration of point-of-care testing solutions is a growth-oriented market segment. Point-of-care diagnostics offer quicker results, fewer diagnostic mistakes, and cost savings. Healthcare providers are investing more in point-of-care diagnostics for gonorrhea and other sexually transmitted infections, allowing for faster treatment and reducing the need for costly laboratory testing.
Collaborations between Manufacturers of Diagnostic Tests and Public Health Agencies
The market is expanding as a result of partnerships between diagnostic test manufacturers and public health agencies. These collaborations involve the development and distribution of innovative new testing solutions. By combining their resources and knowledge, diagnostic test manufacturers and public health agencies can enhance the quality of care for patients and effectively address the expanding threat of gonorrhea.
Implementation of Regulatory Frameworks for Gonorrhea Control
The implementation of regulatory frameworks to combat the gonorrhea epidemic is an additional factor driving market expansion. Governments and regulatory bodies have established frameworks to encourage innovation and investment in gonorrhea testing and treatment. Robust regulatory frameworks for STI testing encourage the development of innovative and dependable testing solutions, thereby propelling market growth.
Latest Trends
NAATs (Nucleic Acid Amplification Tests) for the Detection of Gonorrhea
NAATs have become a significant trend in the Gonorrhea Testing Market, as they provide highly sensitive and specific nucleic acid detection for the diagnosis of gonorrhea. These assays can quickly and non-invasively identify the genetic material of microbes in a variety of samples. NAATs are particularly beneficial for detecting asymptomatic infections, which is driving their market growth.
Dual-Testing Utilization for Multiple STIs
Dual testing, which involves testing a single sample for multiple sexually transmitted infections (STIs) simultaneously, has garnered market traction. This efficient method permits the simultaneous diagnosis of multiple infections. Molecular diagnostic tests have facilitated the testing of multiple infections from a single sample, contributing further to the market's demand for dual-testing.
Test Kits for Private and Convenient Home Use
On the gonorrhea testing market, the availability of home-based testing devices is an emerging development. These devices provide individuals with a private and convenient way to test themselves at home for sexually transmitted infections, including gonorrhea. They provide a discreet and cost-effective means for testing for sexually transmitted infections, resulting in substantial market growth potential.
Integration of Digital Health Platforms for Sexually Transmitted Infection (STI) Education and Self-Testing
Digital health platforms play a substantial role in the gonorrhea testing market, specifically in STI education and self-testing. Mobile applications and online platforms provide information on sexually transmitted infections (STIs), offer self-testing options, and facilitate the monitoring of testing and management procedures. These platforms facilitate the procurement of testing equipment, the retrieval of test results, and the provision of treatment options, thereby revolutionizing the approach to gonorrhea testing.
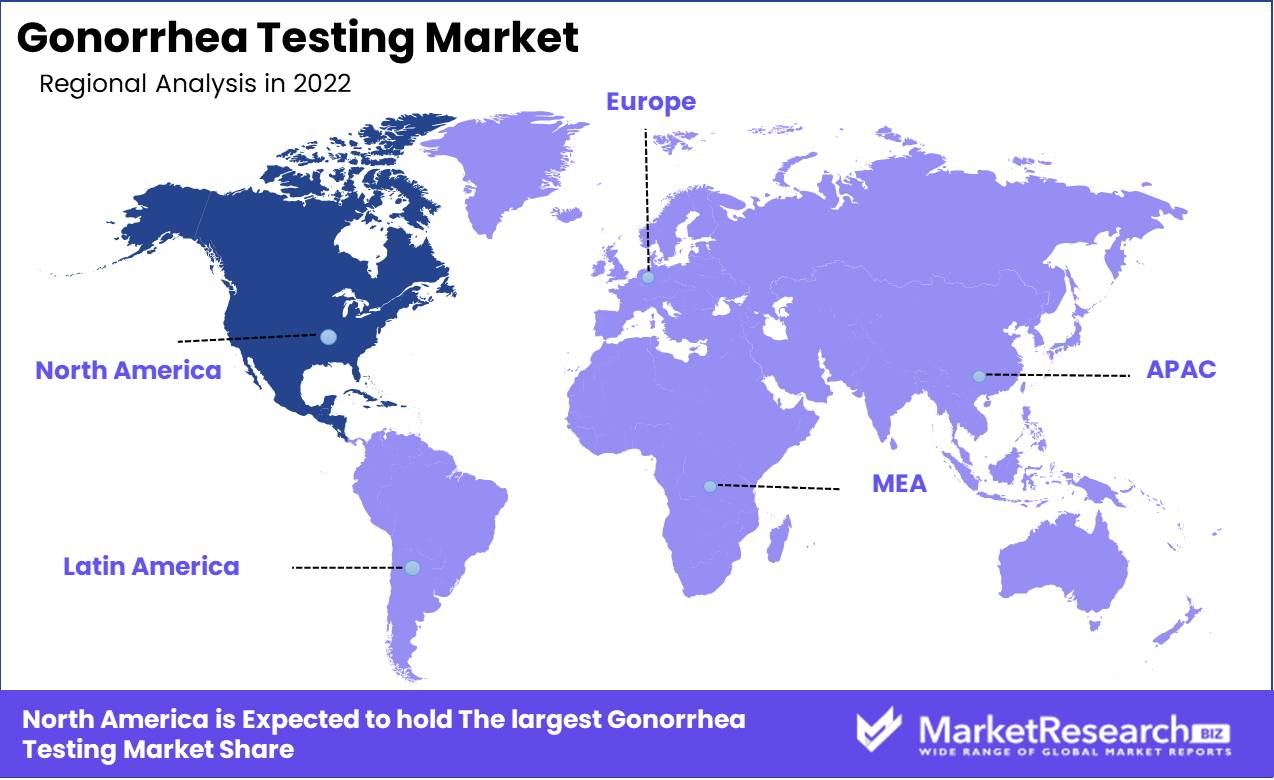
Regional Analysis
The Gonorrhea Testing Market is dominated by North America. The worldwide increase in gonorrhea infections has led to a surge in demand for gonorrhea testing. This indicates that the Gonorrhea Testing Market has been expanding quickly and is projected to continue doing so over the next few years. This article will examine North America's dominance in the gonorrhea testing market. The Gonorrhea Testing Market is presently dominated by North America in terms of revenue and market share. Due to the large prevalence of gonorrhea infections in the United States and Canada, this is the case.
The increase in gonorrhea infections in North America has increased the demand for gonorrhea testing. As a consequence, the Gonorrhea Testing Market in the region has expanded significantly. The market is primarily propelled by technological advances in diagnostics, rising awareness of sexually transmitted infections, and the availability of affordable testing facilities.
North America is home to a number of market leaders in gonorrhea testing. These companies consist of Hologic Inc., Roche Diagnostics, Abbott Laboratories, and Danaher Corporation. The high sensitivity and specificity of the Hologic Aptima Combo 2 and Panther systems and the Roche Diagnostics cobas 4800 system place them among the top gonorrhea testing products in North America. These products have aided to increase North America's aggregate market revenue share.
The United States and Canada have well-established healthcare systems, which has contributed to the expansion of the gonorrhea testing market. Numerous diagnostic tests, such as nucleic acid amplification tests, rapid diagnostic tests, and microfluidic chip-based tests, have contributed to an increase in the number of gonorrhea tests conducted in the region. Additionally, favorable government policies in North America regarding the prevention and control of sexually transmitted infections have significantly contributed to the expansion of the gonorrhea testing market.
The growing understanding of the significance of early detection and treatment of gonorrhea infections has increased the demand for testing services. Healthcare providers in North America are increasingly recommending gonorrhea testing to sexually active individuals, particularly those at a high risk of infection, such as men who have sex with other men and individuals with multiple sex partners.
Key Regions and Countries
North America
- US
- Canada
- Mexico
Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
Gonorrhea is a highly contagious sexually transmitted disease (STD) that, if left untreated, can cause significant and persistent health issues such as infertility and pelvic inflammatory disease. Recent reports indicate that the incidence of gonorrhea is rising swiftly in both developed and developing nations worldwide. This has led to the creation of innovative diagnostic instruments and treatment options.
The global gonorrhea testing market is intensely competitive and is dominated by a handful of companies, including Hologic Inc., Abbott Laboratories, F. Hoffmann-La Roche Ltd, Becton, Dickinson and Company, and Bio-Rad Laboratories. These innovators have enhanced the efficacy of early gonorrhea detection and treatment by developing innovative and highly accurate diagnostic tests.
The market has witnessed a number of new product introductions and strategic partnerships between market leaders to expand their market presence. For example, in 2020, Hologic Inc. introduced its Aptima BV and Aptima CV/TV assays for bacterial vaginosis (BV) and trichomoniasis testing, which can also detect gonorrhea and chlamydia. Additionally, the company has collaborated with global health organizations such as the World Health Organization (WHO) to develop affordable and accessible gonorrhea diagnostic assays.
Top Key Players in Gonorrhea Testing Market
- Abbott Laboratories
- Becton, Dickinson and Co.
- bioMérieux SA
- Bio-Rad Laboratories Inc.
- Cepheid
- Danaher
- DiaSorin S.p.A
- Hologic Inc
- F. Hoffmann-La Roche Ltd.
- Thermo Fisher Scientific Inc.
- Qualigen Technologies Inc.
- Siemens Healthineers
- Other Key Players
Recent Development
In 2021, The FDA granted Abbott Laboratories approval for its BinaxNOW Gonorrhea test. This test is a highly accurate and rapid diagnostic instrument that allows healthcare professionals to effectively diagnose and treat gonorrhea infections. The BinaxNOW Gonorrhea test is expected to revolutionize the diagnosis of this infectious disease, particularly in developing nations where its prevalence is high.
In 2022, the FDA approved Quidel Corporation's Sofia Gonorrhea test, another rapid point-of-care test for gonorrhea detection. The Sofia Gonorrhea test is simple to use and provides healthcare professionals with a rapid and accurate diagnosis. It is especially useful in emergency rooms, clinics, and other contexts that require swift diagnosis.
In 2023, Phase III clinical trials, Roche announced the development of a new molecular test for the detection of gonorrhea. It is anticipated that the new molecular test from Roche will be extremely accurate and sensitive, making it an indispensable instrument for diagnosing and treating this infectious disease. It also has the potential to prevent the spread of gonorrhea by promptly identifying and treating asymptomatic cases.
Report Scope:
Report Features Description Market Value (2022) USD 92 Bn Forecast Revenue (2032) USD 154.5 Bn CAGR (2023-2032) 5.5% Base Year for Estimation 2022 Historic Period 2016-2022 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Test Type (Nucleic Acid Amplification Tests (NAATs), Culture-Based Tests, Gram Stain, Other Test Types)
By Sample Type (Blood, Lymph Fluid, Urine, Throat Swab, Penis/Vaginal Swab)
By End-User (Hospitals and Clinics, Laboratories, Point of Care Testing)Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Abbott Laboratories, Becton, Dickinson and Co., bioMérieux SA, Bio-Rad Laboratories Inc., Cepheid , Danaher , DiaSorin S.p.A, F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific Inc. , Qualigen Technologies Inc., Siemens Healthineers, Other Key Players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Abbott Laboratories
- Becton, Dickinson and Co.
- bioMérieux SA
- Bio-Rad Laboratories Inc.
- Cepheid
- Danaher
- DiaSorin S.p.A
- Hologic Inc
- F. Hoffmann-La Roche Ltd.
- Thermo Fisher Scientific Inc.
- Qualigen Technologies Inc.
- Siemens Healthineers
- Other Key Players




