
Flu Treatment Market By Flu Type (Flu A, Flu B & Others), By Treatment(Oseltamivir Phosphate, Zanamivir & Others), By Distribution Channel(Hospital Pharmacies, Retail Pharmacies & Other), By Region And Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, And Forecast 2023-2032
-
37575
-
June 2023
-
160
-
-
This report was compiled by Correspondence Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Quick Navigation
Report Overview
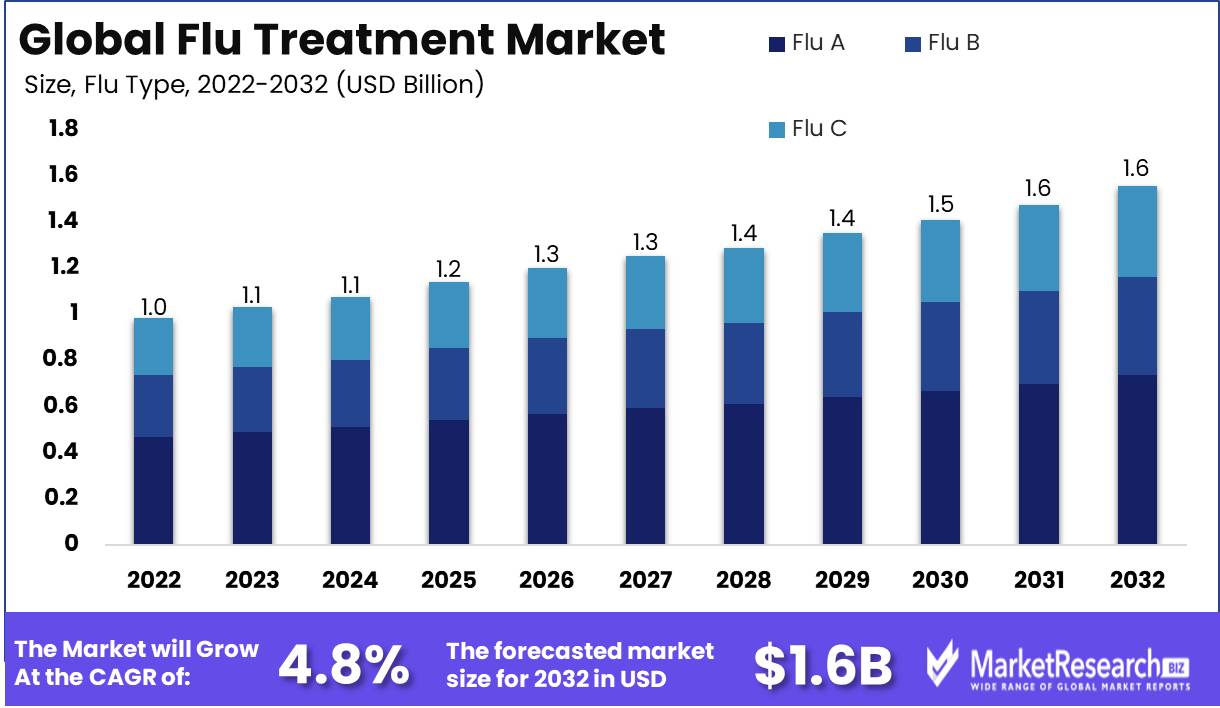
Flu Treatment Market size is expected to be worth around USD 1.6 Bn by 2032 from USD 1.0 Bn in 2022, growing at a CAGR of 4.8% during the forecast period from 2023 to 2032.
The perpetual expansion of the global flu treatment market is a testament to the relentless pursuit of innovative breakthroughs and pioneering technologies to meet the rising demand. This report aims to provide a comprehensive overview of this thriving market, shedding light on its paramount significance, myriad advantages, notable innovations, substantial investments, impressive growth trajectory, diverse industries investing, growth drivers, ethical dilemmas, responsible employment, and diverse business applications.
The global flu treatment market consists of a thriving industry devoted to the development of remedies, vaccines, and medications designed to combat influenza and its associated diseases. Influenza, a highly contagious respiratory illness that frequently causes severe complications, affects millions of people worldwide. Consequently, the market plays a crucial role in mitigating the effects of influenza outbreaks and pandemics, exerting substantial influence over the administration of public health.
Unquestionably, the global flu treatment market is of utmost importance for protecting the health of individuals around the globe from the debilitating effects of influenza. By providing an array of treatment options including antiviral drugs targeting flu symptoms, preventative vaccines, and specialized medicines tailored to combat specific flu strains such as H1N1, this market demonstrates its indispensability during flu outbreaks and pandemics, when the rapid development and expeditious distribution of effective treatments can be the difference between life and death for countless people.
The proliferation of innovative technologies and products in the global flu treatment market over the years is a testament to its evolutionary nature. The development of RNA-based vaccines that provide rapid responses to emergent flu strains and significantly reduce the time required for vaccine production and distribution is a significant innovation in this field. Moreover, the market has witnessed remarkable improvements in the efficacy of flu vaccines as a result of the creation of more potent antigen combinations.
Enthusiasm for the global flu treatment market has attracted substantial investments from a variety of stakeholders, including pharmaceutical companies, governmental organizations, and research institutions, thereby fostering the development of new, more effective treatments, as well as enhancements to existing options, thereby making them more accessible and affordable. Notably, flu treatment options have been incorporated into an array of products and services, such as telemedicine and urgent care clinics, thereby increasing their availability to the general public.
Driving factors
High Prevalence of Influenza and Increasing Demand for Flu Treatments
In the near future, the global flu treatment market is anticipated to expand significantly. This expansion can be attributed to a number of important factors. First, the high prevalence of influenza, which annually affects millions of people around the globe, has caused a surge in demand for flu treatments. Seasonal influenza outbreaks are becoming more frequent and widespread, increasing the demand for effective treatments.
Advancements in Antiviral Medications and Vaccines
Advancements in antiviral drugs and vaccines have had a positive impact on the flu treatment market. Constant efforts in research and development have resulted in the introduction of new treatments and therapies, resulting in improved flu prevention and treatment. These innovations have significantly increased patient recovery time and decreased complications. As a consequence of their increased effectiveness, there is a growing demand for flu treatment products.
A Growing Awareness of Flu Prevention and Treatment
Awareness of the importance of flu prevention and treatment is another important factor driving the expansion of the flu treatment market. As more people become aware of the dangers of influenza and the necessity of seeking prompt treatment when ill, it is anticipated that the demand for flu treatments will continue to increase. This increased awareness encourages individuals to prioritize their health and take the necessary precautions.
Restraining Factors
Antiviral Resistance Concerns
Antiviral drugs are frequently used to treat influenza infections. However, the development of antiviral resistance is a major concern for healthcare professionals. When the influenza virus acquires genetic modifications that enable it to resist antiviral medications, antiviral resistance occurs. In recent years, pervasive antiviral resistance to the influenza A (H1N1) virus strain has been reported, which is cause for grave concern. The emergence of antiviral resistance can reduce the efficacy of current antiviral remedies, making it more difficult to control influenza outbreaks.
Potential Side Effects of Flu Treatments
All medications have the potential for adverse effects, and influenza treatments are no exception. Antiviral medications frequently cause nausea, vomiting, diarrhea, headaches, and vertigo. In uncommon instances, some antiviral medications have been linked to more severe adverse effects, including confusion, hallucinations, and seizures. These adverse effects can restrict the use of antiviral medications, particularly in patients with preexisting medical conditions.
Flu Type Analysis
Due to its high transmission rate, which makes it a top concern for research and development, the Flu A Segment dominates the global flu treatment market. There are three varieties of influenza viruses: A, B, and C, with type A being the most prevalent. These viruses are known for their constant evolution and change, which means they are capable of mutating into new strains that require specialized treatments. The 2009 H1N1 pandemic, which caused over 200,000 fatalities worldwide and led to the development of Tamiflu and Relenza, was caused by the Flu A Segment.
The adoption of the Flu A Segment has been driven by economic development in emerging economies. As these nations invest in their healthcare infrastructure and enhance their access to medical services, the demand for effective influenza treatments increases. In recent years, emerging economies such as China, India, Brazil, and South Africa have experienced substantial economic expansion. This has resulted in an increase in discretionary income and a demand for improved healthcare services, which has led to the widespread adoption of Flu A Segment in these markets.
Treatment Analysis
The Oseltamivir Phosphate Segment dominates the global flu treatment market due to its efficacy in reducing flu symptoms and the risk of complications. It is an antiviral drug used to treat and prevent influenza A and B strains. In many countries, Oseltamivir Phosphate is prescribed as the first-line treatment for influenza because of its ability to reduce the duration and severity of flu symptoms.
The adoption of the Oseltamivir Phosphate Segment was spurred by the economic development of emerging economies. As the economies of these nations develop, so does the demand for improved healthcare services and treatments. This increased demand for influenza treatments has increased the use of the Oseltamivir Phosphate Segment. Trends and behaviors of consumers toward the Oseltamivir Phosphate Segment also contribute to its dominance in the global flu treatment market. Consumers prefer flu treatments that not only alleviate symptoms but also reduce the likelihood of severe complications. Oseltamivir Phosphate Segment is highly effective at attaining these objectives and has become a popular treatment choice among many patients.
Distribution Channel Analysis
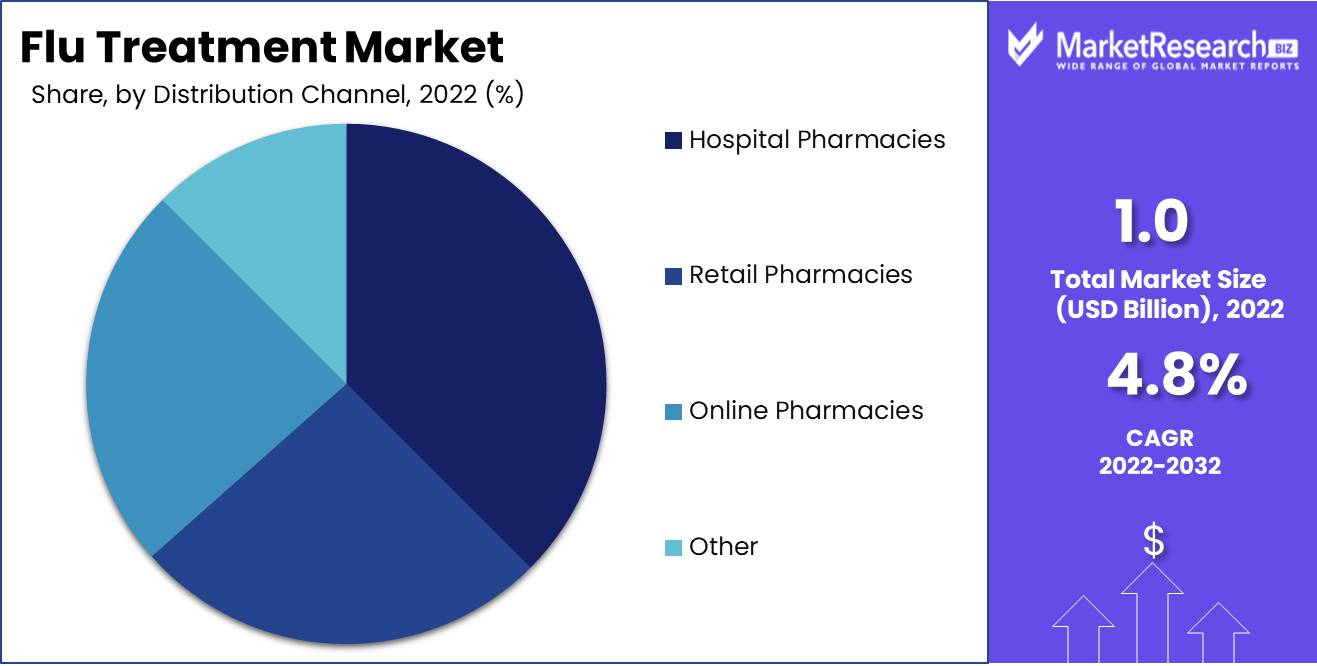
The Hospital Pharmacies Segment dominates the worldwide flu treatment market due to its ability to provide specialized medicinal items and support healthcare services. This segment provides hospitalized patients with a variety of flu treatments, including antiviral medications and flu vaccines. Due to its dependability in supplying hospitals with high-quality medical products and services, the hospital pharmacies Segment is the preferred distribution channel in many nations.
In emerging economies, the adoption of the Hospital Pharmacies Segment has been driven by rising economic growth and investments in healthcare infrastructure. The expansion of hospitals in these nations to meet the rising demand for healthcare services has led to a rise in the adoption of the Hospital Pharmacies Segment. The increase in private healthcare providers has also contributed to this segment's growth.
Key Market Segments
By Flu Type
- Flu A
- Flu B
- Flu C
By Treatment
- Oseltamivir Phosphate
- Zanamivir
- Peramivir
- Baloxavir Marboxil
- Other
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Other
Growth Opportunity
Next-Generation Flu Vaccines and Antiviral Therapies
The development of next-generation flu vaccines and antiviral therapies has been a key factor in the global flu treatment market's growth. Next-generation flu vaccines are anticipated to be more effective against a broader spectrum of seasonal and pandemic flu viruses and to have a longer duration of protection. Antiviral therapies, on the other hand, are used to treat individuals infected with the influenza virus and can lessen the severity of the disease and prevent complications.
The increasing demand for next-generation flu vaccines and antiviral therapies has prompted the pharmaceutical industry to conduct more research and development. Numerous pharmaceutical companies are making substantial investments in the research and development of innovative flu vaccines and antiviral therapies, which is anticipated to drive the growth of the global flu treatment market in the coming years.
Expansion of Flu Vaccination Campaigns
The expansion of flu vaccination campaigns has contributed significantly to the growth of the global flu treatment market. Governments and healthcare organizations around the world are promoting vaccination campaigns to increase the number of people who are immunized against the influenza virus and raise awareness of the significance of flu vaccination.
In addition, the growing trend of employer-sponsored vaccination programs is anticipated to boost the growth of the global flu treatment market. As part of their wellness programs, many employers provide free or subsidized flu vaccines to their employees, which is expected to increase the adoption of flu vaccines and contribute to the growth of the market.
Research on Universal Flu Vaccines
The global flu treatment market is also focused on the development of universal flu vaccines, which are intended to provide protection against all variants of influenza virus. Universal flu vaccines have the potential to transform how we prevent and treat influenza viruses.
Currently, flu vaccines must be reformulated annually to match the strains of the virus anticipated to circulate during flu season. However, universal flu vaccines would provide protection against all strains of the influenza virus, thereby eliminating the need for annual reformulation.
Latest Trends
Adoption of Quadrivalent and High Dose Flu Vaccines
Vaccination remains the primary method for preventing the flu worldwide. The effectiveness of influenza vaccines in reducing the incidence of flu has been demonstrated for over 70 years. However, as the influenza virus evolves, so does the influenza vaccine. Manufacturers have developed quadrivalent and high-dose flu vaccines in response to the increasing demand for improved vaccines.
Quadrivalent influenza vaccines are intended to provide protection against four distinct influenza viruses, including two influenza A viruses and two influenza B viruses. High-dose influenza vaccines, on the other hand, contain four times as many antigens as the standard vaccine and are recommended for individuals 65 years and older. The global adoption of quadrivalent and high-dose influenza vaccines is increasing, and this is a significant trend influencing the influenza treatment market. These vaccines are preferred by many people because they are more efficacious and have fewer side effects.
Utilization of Antiviral Drugs for Treatment and Prophylaxis
Antiviral medications are another essential component of influenza treatment. They function by inhibiting the virus' replication, thereby diminishing the severity and duration of symptoms. Antiviral medications can also be employed for prophylaxis and prevention. In recent years, the use of antiviral medications for the treatment and prevention of influenza has increased significantly. Popular antiviral medications for influenza include oseltamivir, zanamivir, and peramivir. These medications reduce the duration and severity of influenza symptoms and are widely prescribed by physicians around the globe.
Telemedicine for Remote Flu Diagnosis and Consultation
Telemedicine is the use of technology to deliver healthcare services remotely. In recent years, telemedicine has gained popularity as it provides patients with convenient and cost-effective healthcare services. Telemedicine is especially useful for the diagnosis and treatment of influenza, as patients can receive remote consultations and prescriptions without having to visit a physician in person.
Globally, the use of telemedicine for the diagnosis and treatment of influenza has increased. Patients can now access healthcare services from the convenience of their homes, workplaces, or any other location, thanks to the proliferation of smartphones and other mobile devices.
Regional Analysis
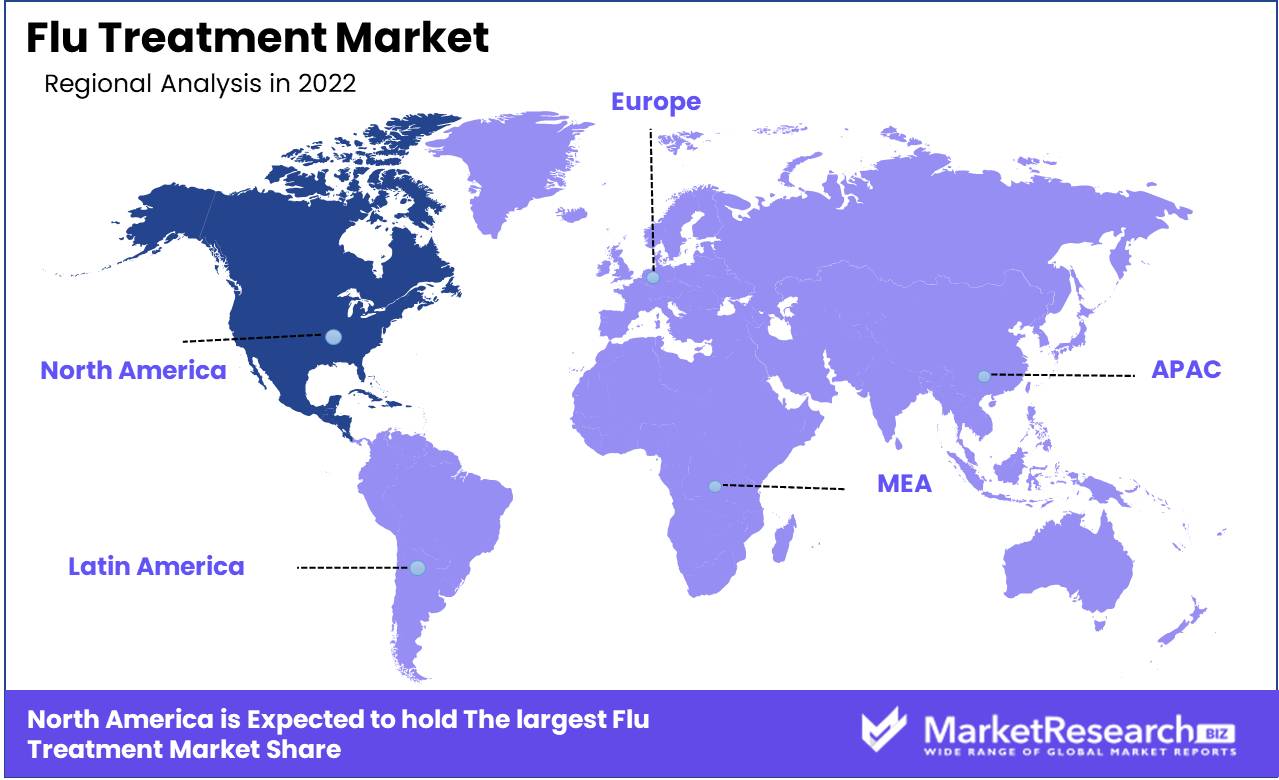
The North American region's global dominance over the flu treatment market plays a significant role in influencing the industry. North America is a region renowned throughout the globe for its advanced healthcare infrastructure and cutting-edge medical facilities. In addition, the region is home to some of the world's most competent and established pharmaceutical companies. Due to the development of research and development facilities, quick regulatory programs, and rising demand for cutting-edge medical therapies, the North American flu treatment market continues to expand.
Antiviral drugs and vaccines dominate the North American flu treatment market. Antiviral drugs are used to treat and prevent the worsening of influenza symptoms. These drugs work by inhibiting the virus' replication and allowing the immune system to battle the disease. Current antiviral treatments for influenza include Oseltamivir and Baloxavir Marboxil, among others.
Vaccines, on the other hand, stimulate the immune system to produce antibodies against the flu virus, making them a crucial aid in preventing the flu. The North American influenza vaccine market is significant because it effectively combats the proliferation of influenza throughout the region. Trivalent and quadrivalent influenza vaccines are the two most common varieties. The trivalent vaccine protects against three strains of the influenza virus, while the quadrivalent vaccine protects against four strains.
The North American flu treatment market is intensely competitive and dynamic. Hundreds of pharmaceutical companies are continually vying for industry leadership positions. In addition, companies invest heavily in research and development to enhance existing products and develop innovative, safe, cost-effective, and effective drugs. The robust development of the flu treatment market is anticipated to continue in the coming years, with medical progress and technological innovation among the factors driving this growth.
Key Regions and Countries
North America
- US
- Canada
- Mexico
Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
In recent years, the increase in incidence and prevalence of the influenza virus has propelled the accelerated growth of the global flu treatment market. The main players in this market are pharmaceutical companies specializing in the development and production of drugs for the treatment of influenza.
F. Hoffmann-La Roche AG is one of the leading players on the global market for influenza treatments. Due to its comprehensive product portfolio, which includes the popular influenza drug Tamiflu, the company has a strong presence on the market.
GlaxoSmithKline plc is an additional significant player in the global market for influenza treatments. Relenza and Fluarix have extensively used products for the treatment of influenza offered by the company.
In addition to being a prominent player in the global flu treatment market, Abbott Laboratories focuses on developing new and innovative drugs for the treatment of influenza. The company's portfolio of products includes Amantadine and Rimanadine.
Top Key Players in Flu Treatment Market
- Hoffmann-La Roche AG
- Novartis AG
- Sanofi
- Teva Pharmaceutical Industries Ltd
- AstraZeneca Plc
- Daiichi Sankyo Company
- BioCryst Pharmaceuticals, Inc
- NATCO Pharma Limited
- Bristol-Myers Squibb Company
- Other
Recent Development
- In 2021, the pharmaceutical company Novartis made a significant advancement in the treatment of influenza. The US Food and Drug Administration (FDA) has approved the company's new flu treatment, Xofluza.
- In 2022, The pharmaceutical companies GlaxoSmithKline and Sanofi announced a partnership to develop a new influenza vaccine that is intended to be more effective than current vaccines. mRNA, a form of genetic material, will be utilized in the development of the vaccine.
- In 2023, the pharmaceutical company Pfizer will initiate a clinical trial of its novel influenza vaccine. The vaccine is designed to protect against four distinct influenza virus variants, making it potentially more effective than other flu vaccines currently on the market.
Report Scope
Report Features Description Market Value (2022) USD 1.0 Bn Forecast Revenue (2032) USD 1.6 Bn CAGR (2023-2032) 4.8% Base Year for Estimation 2022 Historic Period 2016-2022 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Flu Type(Flu A, Flu B, Flu C), By Treatment(Oseltamivir Phosphate, Zanamivir, Peramivir, Baloxavir Marboxil, Other), By Distribution Channel(Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, Other) Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Norbord Inc., Kronospan Limited, West Fraser Timber Co. Ltd., Timber Products Company, Weyerhaeuser Company, Georgia-Pacific LLC, Bucina DDD, spol. s r.o. (Ltd.), Sonae Indústria, Freres Lumber Co., Inc., Dongwha Enterprise Co., Ltd., Kastamonu Entegre, Hampton Affiliates, Duratex Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Hoffmann-La Roche AG
- Novartis AG
- Sanofi
- Teva Pharmaceutical Industries Ltd
- AstraZeneca Plc
- Daiichi Sankyo Company
- BioCryst Pharmaceuticals, Inc
- NATCO Pharma Limited
- Bristol-Myers Squibb Company
- Other




