
eClinical Solutions Market By Product Type (Electronic data capture, Clinical data management systems, and Others), By Delivery Mode (Web-hosted, Licensed enterprise, Cloud-based solutions), By clinical trial (Phase I, Phase II, and Other), By Region And Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, And Forecast 2023-2032
-
562
-
May 2023
-
163
-
-
This report was compiled by Trishita Deb Trishita Deb is an experienced market research and consulting professional with over 7 years of expertise across healthcare, consumer goods, and materials, contributing to over 400 healthcare-related reports. Correspondence Team Lead- Healthcare Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Quick Navigation
Report Overview
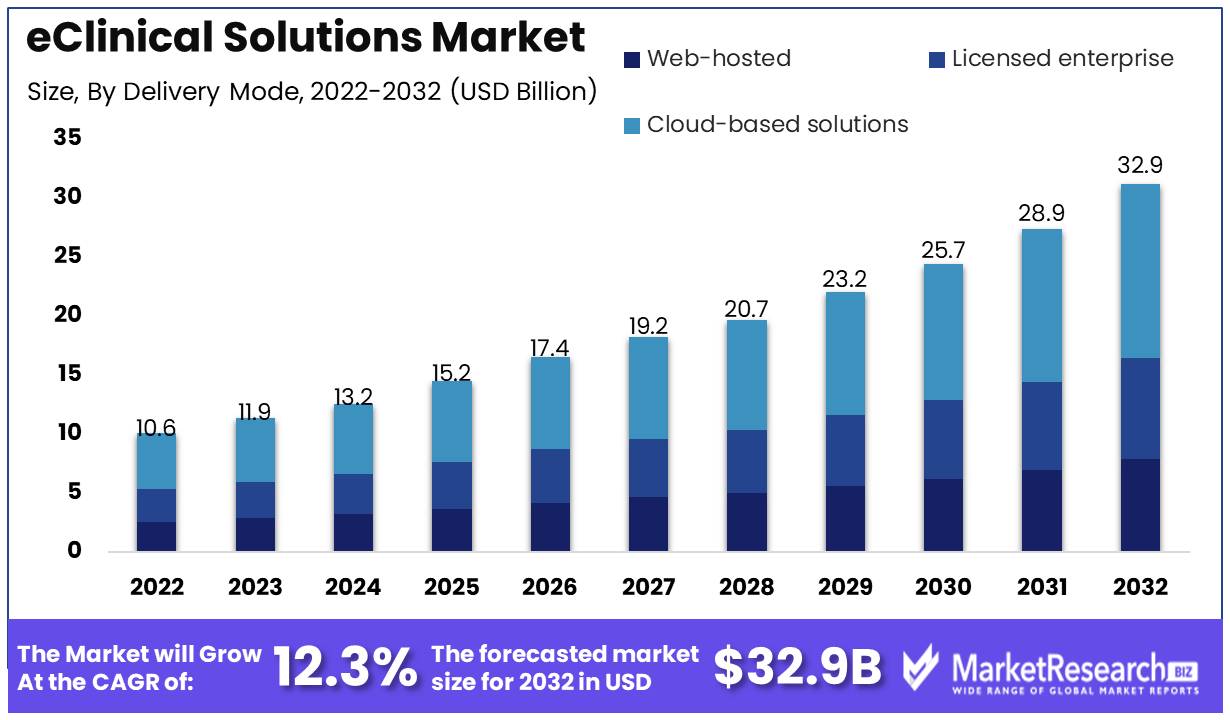
eClinical Solutions Market size is expected to be worth around USD 32.9 Bn by 2032 from USD 10.6 Bn in 2022, growing at a CAGR of 12.3% during the forecast period from 2023 to 2032.
The eClinical Solutions Market has experienced a rapid expansion as numerous industries have invested in this innovative technology. eClinical Solutions incorporate the use of electronic tools and technology to improve the efficacy and precision of clinical trials, data management, and statistical analysis. In this article, we will discuss the eClinical Solutions Market's definition, objectives, significance, advantages, notable innovations, significant investments, and incorporation into products and services. We will examine the expansion and applications of the eClinical solutions market in forecast period.
eClinical Solutions entail the application of electronic tools, medical device and technology to enhance the effectiveness and precision of clinical trials, data management, and analysis. These solutions consist of electronic data capture (EDC), clinical data management systems (CDMS), electronic patient-reported outcomes (ePRO), randomization and trial supply management (RTSM), and clinical trial management systems (CTMS).
The primary objective of eClinical solutions is to improve the efficacy of clinical trials by streamlining data acquisition and reducing the time and costs associated with conducting research. These solutions allow clinical researchers to spend less time on data collection and analysis, so they can devote more time to scientific research and analysis.
E-Clinical Solution are significant due to their numerous advantages over conventional research methods. They increase the speed and efficacy of data collection and analysis, reduce the risk of errors, improve the accuracy and validity of data, and provide real-time access to data, thus accelerating research outcomes.
With the healthcare sector embracing the digital age, e-clinical solutions are at the forefront of this revolution, contributing significantly to the sector's financial success. eCOA, represents a revolutionary shift in patient-reported outcomes. Traditionally, patients would record their experiences on paper, leading to delays and potential data loss. eCOA platforms digitize this process, allowing patients to report their symptoms, side effects, and quality of life directly through user-friendly interfaces.
Data privacy and security is a concern associated with e-clinical solutions from an ethical standpoint. To protect the confidentiality, administration, and sharing of data for both research participants and investigators, it is essential to implement data management and security policies. Ensuring patient safety is paramount in the market. Innovative safety solutions, such as real-time monitoring and adverse event reporting, are gaining prominence to minimize risks associated with these devices.
Driving factors
Streamlining Patient Recruitment and Information Access
The market size for eClinical Solutions has been a game-changer in clinical research studies. Patient recruitment and retention have always been significant obstacles in clinical trials, which can affect the duration and cost of the study. Nonetheless, with the implementation of eClinical solutions, researchers can streamline patient enrollment and increase retention. In clinical research, manual data generation and lack of real-time data access have also been significant pain points. However, eClinical solutions provide real-time data access, making the process more efficient and cost-effective. Real-time data access is critical in healthcare settings, particularly in patient monitoring and medical device management. Healthcare professionals can receive real-time updates on patients' vital signs, ensuring immediate intervention if necessary.
Enhanced Productivity and Cost Effectiveness
Researchers increasingly favor eClinical solutions for their increased efficacy and cost effectiveness. These solutions facilitate the research process and improve site performance. These solutions are being adopted by Medical Device Manufacturers, pharmaceutical companies and independent researchers in order to eliminate procedural bottlenecks and streamline the clinical trial process. CROs and biotech companies have significantly increased their adoption of eClinical solutions in recent years.
Compliance Assistance for eClinical Solutions
Regulatory alterations affecting the market for eClinical Solutions are always possible. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are currently encouraging the adoption of eClinical solutions and streamlining the approval process. These modifications may have a positive effect on the market in forecast period.
Source refers to the point where patient data originates. It serves as the foundation of clinical research. eClinical Solutions leverage Source data integration to ensure data accuracy and consistency. By capturing patient data directly at the source, such as electronic health records (EHRs) and medical devices, researchers eliminate transcription errors and ensure real-time access to critical information.
Restraining Factors
High Costs of Implementation
The high cost of implementation is one of the major restraining factors for eClinical solutions. Typically, the implementation of eClinical solutions requires a substantial investment in software licenses, hardware, and IT support services. Many government and healthcare organizations, particularly modest businesses, find it difficult to designate the resources required to implement these solutions. The high implementation costs are also a major factor that prevents new competitors from entering the market.
Price of Technical Support for Cloud-Based Applications
The price of technical support for cloud-based solutions is an additional cost factor. Due to their adaptability and scalability, cloud-based solutions have grown in popularity, making them an attractive option for many government and healthcare organizations. However, cloud-based solutions require technical expertise, which can be costly to obtain. Organizations that lack the resources to implement cloud-based solutions are compelled to implement costly on-premise alternatives. In such instances, the cost of technical support may pose a substantial barrier to the adoption of cloud-based solutions.
Product Type Analysis
Electronic Data Capture (EDC) dominate eClinical Solutions market segment by products type. With the development of technological advancements, the market for eClinical solutions has been revolutionized, and electronic data capture (EDC) is one of the market's dominant segments. EDC is the process of electronically accumulating clinical trial data, which includes data management, analysis, and presentation. The pharmaceutical and biotechnology industries have employed Electronic Data Capture for the efficient administration of clinical trials. Electronic Data Capture solutions are playing a pivotal role in urology clinical trials by streamlining data collection and management. These tools enhance efficiency, reduce errors, and accelerate the product development by providing real-time access to patient data.
Electronic Data Capture's cost-effectiveness, time-saving, accuracy, and data quality have driven its adoption. Due to its real-time data monitoring and administration, the Electronic Data Capture category will dominate eClinical solutions. Electronic Data Capture can help pharmaceutical and biotechnology companies manage and process enormous amounts of data in real time to improve clinical trial efficiency. Consumers are adopting new healthcare technology. Electronic Data Capture is a popular strategy for clinical trial data collection and management. Electronic Data Capture's accuracy and data quality have encouraged customer acceptance. Consumers favor Electronic Data Capture because to its cost-effectiveness, convenience of access, and data management.
Delivery Mode Analysis
The segment of cloud-based eClinical solutions dominates the eClinical solutions market. One of the leading market segments in the eClinical solutions is Cloud based eClinical solutions. It provides a scalable and secure platform for centrally managing clinical trial data. The cloud-based delivery mode offers a number of advantages, including cost-effectiveness, security, and reduced infrastructure needs. Clinical trial management software is extensively used in the pharmaceutical and biotechnology industries.
Cloud-based eClinical solutions are popular because of their cost-effectiveness, scalability, and accessibility. Consumers choose cloud-based eClinical solutions for clinical trial data management. Consumers choose these solutions because to their better data quality and faster processing. Due to many factors, cloud-based eClinical solutions are expected to develop the quickest. This segment is growing due to the healthcare sector's adoption of digital technologies, the pharmaceutical and biotechnology industries' adoption of cloud-based solutions, and emerging economies' economic growth. Cloud-based eClinical solutions manage clinical trial data cost-effectively, securely, and scalablely.
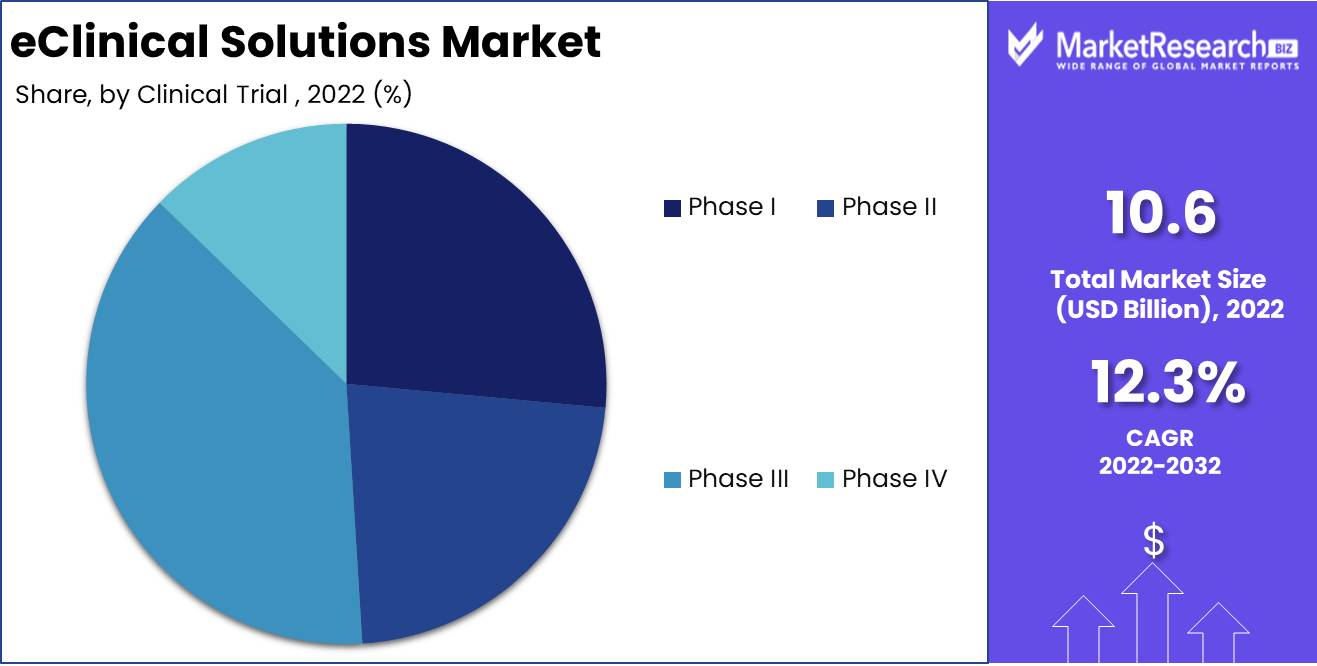
Clinical Trial Phase Analysis
Marked eClinical Solutions Segment Dominates Phase III Clinical Trials. The Clinical Trial Phase III clinical trials segment dominates the market for eClinical solutions. The final and most crucial phase of clinical trials is Clinical Trial Phase III. The success of these trials determines whether regulatory authorities will approve a novel drug or therapy. eClinical solutions are utilized by the pharmaceutical and biotechnology industries for the administration of Clinical Trial Phase III clinical trials due to their cost-effectiveness, quicker data processing, and enhanced data quality.
Consumers like cutting-edge healthcare technology. Due to their cost-effectiveness, speed, and data quality, e-clinical solutions are commonly used to manage Clinical Trial Phase III clinical studies. Consumers prefer these solutions over manual, error-prone clinical trial management. Due to various factors, Phase III clinical trials are expected to increase the fastest. This segment is growing due to the healthcare sector's adoption of digital technologies, the pharmaceutical and biotechnology industries' adoption of e-clinical solutions, cost-effectiveness, faster data processing, and improved data quality, and economic development. E-clinical solutions for Phase III clinical trials are projected to become the standard in forecast period.
End Users Analysis
Contract Research Organizations (CROs) Segment Dominates eClinical Solutions Segment. Contract Research Organizations (CROs) is the leading segment in the market for eClinical solutions. CROs play a crucial role in clinical trial administration, and they require a centralized platform for trial data management. E-clinical solutions offer a cost-effective and expedient way to manage clinical trials. Contract Research Organizations (CROs)are essential partners for urology device manufacturers. They offer expertise in regulatory compliance, clinical trial management, and data analysis, helping companies navigate complex approval processes efficiently.
Consumers prefer Contract Research Organizations -provided e-clinical data management solutions. Contract Research Organizations' cost-effectiveness and efficiency have improved customer acceptance. This category has grown due to global consumer adoption of cutting-edge digital health technology. Due to many factors, Contract Research Organizations (CROs) are expected to develop the quickest. This area is rising due to digital technology usage in healthcare, Contract Research Organizations' embrace of e-clinical solutions, cost-effectiveness, efficiency, centralized data administration, and economic development. The trend should continue in forecast period.
Key Market Segments
By Product Type
- Clinical analytics platforms
- Electronic data capture
- Clinical data management systems
- Safety solutions
- Randomization & trial supply management
- Electronic clinical outcome assessment
- Clinical data integration platforms
- Clinical trial management systems
- Electronic trial master file
- Regulatory information management solutions
By Delivery Mode
- Web-hosted
- Licensed enterprise
- Cloud-based solutions
By Clinical Trial
- Phase I
- Phase II
- Phase III
- Phase IV
By End Users
- Pharmaceutical
- Biopharmaceutical companies
- Contract research organizations
- Consulting service companies
- Medical device manufacturers
- Hospitals
- Academic research institutions
Growth Opportunity
Technological advances are transforming the healthcare industry
In today's world, technological advancements have had a profound effect on every aspect of our existence, including the healthcare industry. With the proliferation of electronic devices, the demand for digital medical solutions has steadily increased. eClinical solutions is one sector of the healthcare industry that has benefited significantly from technological advancements. This industry has experienced a significant increase in demand due to the numerous benefits it provides to healthcare providers and patients in forecast period.
Structure of Regulation and Safety Monitoring
In developed economies such as the United States, the growth of eClinical solutions is driven by regulatory framework and safety surveillance. Due to stringent regulatory structures, eClinical solutions have become increasingly popular in developed economies such as the United States. This has increased the demand for eClinical solutions that are compliant with regulatory requirements. Urology device manufacturers are under strict scrutiny to meet patient compliance and regulatory compliance. Staying compliant with evolving regulations is crucial to market entry and product success.
Data Sharing and Tightening Registration Requirements for Clinical Trials
The US Health Department and NIH are strengthening registration regulatory requirements for clinical trials and fostering data sharing. In order to ensure the safety and efficacy of healthcare products, the US Department of Health and the National Institutes of Health (NIH) have implemented measures to tighten clinical trial registration regulatory requirements and promote data sharing. This action has increased demand for eClinical solutions that can assist healthcare providers in complying with these regulations.
Latest Trends
Surge in Drug Development Studies
The eClinical Solutions Market is experiencing rapid expansion due to a number of factors. An increase in the number of drug development studies conducted worldwide is one of the primary drivers. Pharmaceutical companies are significantly investing in research and development, resulting in a surge in demand for eClinical solutions to streamline and accelerate the clinical trial process.
Increasing Clinical Trials Boost
Alongside the increase in drug development studies, there has been an increase in clinical trials. Increased funding, technological advancements, and the development of novel remedies have all contributed to this expansion. Consequently, the demand for eClinical solutions that improve the efficacy forecast period and precision of clinical trials has increased significantly.
Increasing Acceptance in Clinical Research
In clinical research, the use of eClinical solutions has steadily increased. These solutions streamline and automate clinical trial procedures, rendering them more efficient and cost-effective. In addition, eClinical solutions enhance the precision and dependability of clinical data, which is essential for obtaining regulatory approval for new pharmaceuticals and therapies.
Systems for Clinical Data Management (CDMS)
In the future years, clinical data management systems (CDMS) are anticipated to dominate the market for eClinical solutions. CDMS applications are designed to acquire, store, and manage clinical data from multiple sources. They facilitate the accumulation and analysis of data, allowing researchers to identify trends, monitor patient outcomes, and make informed decisions regarding drug development and clinical trial design.
CDMS Integration with Other eClinical Instruments and Solutions
Integration of CDMS with other eClinical tools and solutions is an emerging trend in the eClinical Solutions Market. During clinical trials, CDMS can be seamlessly incorporated with electronic data capture (EDC) systems, which capture data electronically. Additionally, CDMS can be integrated with electronic patient reported outcomes (ePRO) systems, allowing patients to electronically report symptoms and other health-related data. This integration improves the efficacy and effectiveness of clinical research data collection and analysis.
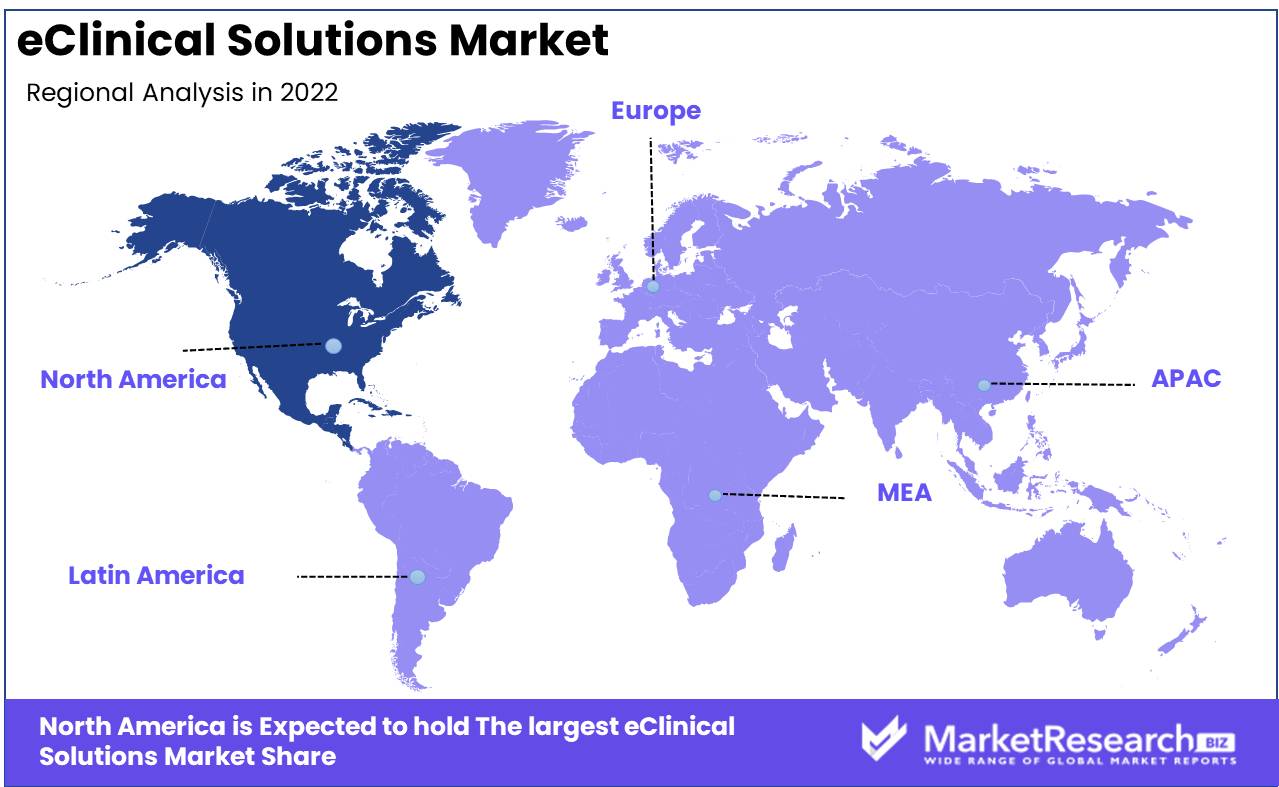
Regional Analysis
The eClinical Solutions Market is led by North America. Extensive research on the global market for eClinical solutions reveals that North America currently dominates the industry. Recent studies indicate that, North America will account for over 51% of the market share, making it the largest revenue source generator on the global eClinical solutions market. This dominance is largely attributable to the increasing number of clinical trials conducted in North America. Several of the world's most influential pharmaceutical companies and clinical research organizations are headquartered in the United States and Canada. These companies are investing significantly in the research and development of eClinical solutions to streamline and improve the efficacy of their clinical trials.
The high level of technology adoption in North America is another important factor driving the region's growth in the eClinical solutions market. It is the ideal environment for deploying eClinical solutions due to its sophisticated infrastructure and high-speed internet capabilities. The adoption of cloud-based technology is also acquiring prominence in this region. This is a result of the increasing demand for remote monitoring and access to real-time data, which has been exacerbated by the COVID-19 pandemic in North America.
Positive government initiatives in North America have contributed to the expansion of eClinical solutions in the region. In the United States, for instance, the 21st Century Cures Act encourages the use of electronic health records and eClinical solutions, thereby fostering market expansion.
In North America, the eClinical solutions market is further segmented into electronic data capture (EDC), clinical trial management system (CTMS), clinical analytics platform (CAP), and randomization and trial supply management (RTSM). Due to the increasing demand for real-time data sharing and analysis, EDC is the most prominent of these segments.
Key Regions and Countries
North America
- US
- Canada
- Mexico
Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
In recent years, the market for eClinical solutions has expanded significantly as the healthcare industry increasingly employs technological solutions to enhance patient care and healthcare outcomes. In this swiftly transforming industry, the main key market players in the eClinical solutions market are driving innovation and establishing a reputation for themselves with their cutting-edge technological offerings.
Oracle Corporation is one of the most prominent participants in this market. Oracle's eClinical solutions enable users to effortlessly manage complex clinical trial workflows and data streams while maintaining data quality and precision. In addition, it provides clinical data management and electronic data capture (EDC) services.
Merge Healthcare's software is used by over 7,000 healthcare government and organizations in over 50 countries. The Merge Health Incorporated company's customers include hospitals, imaging centers, specialty clinics, clinical research institutes, and pharmaceutical firms. In 2015, Merge Healthcare was acquired by IBM. In 2022, IBM spun off its Watson Health business unit, which included Merge Healthcare, into a new company called Merative.
Parexel International Corporation provides a comprehensive range of services for all phases of clinical development, from early-stage clinical trials to late-stage regulatory submissions. Parexel International Corporation also offers a variety of innovative solutions to help its clients streamline the clinical development process and bring new treatments to market faster.
Datatrak International, Inc. is a company that specializes in providing technology solutions for clinical trial management and electronic data capture (EDC). Founded in 1991 and headquartered in Mayfield Heights, Ohio, Datatrak has established itself as a leading player in the clinical research and healthcare technology sectors.
Top Key Players in Eclinical Solutions Market
- Parexel International Corporation
- Oracle Corporation
- Merge Health Incorporated
- Datatrak International, Inc.
- BioClinica
- CRF Health
- ERT
- E-Clinical Solutions
- OmniComm Systems Inc
- Medidata Solution
Recent Development
In July 2022, Oracle Corporation partnered with Microsoft to provide Azure clients with Oracle Cloud Infrastructure databases. This cooperation will provide eClinical Solutions Market organizations a simplified, cloud-based data solution.
IDDI distributes Clario, a leading Randomization and Trial Supply Management (RTSM) Software as a Service provider. Clario can reach new consumers and provide IDDI's clients with unique and effective solutions by expanding their offerings.
In April 2021, Datatrak International, Inc announced Datatrak Direct, an all-in-one iOS and Android data gathering software for ePRO, eCOA, and eConsent. This software allows patients to simply input and submit data to their healthcare professionals, revolutionizing the business.
In February 2021, Parexel a biopharmaceutical research business, teamed with NeoGenomics to improve cancer clinical trial patient selection. This will increase precision medicine in these clinical trials and provide researchers better data to cure cancer.
Report Scope:
Report Features Description Market Value (2022) USD 10.6 Bn Forecast Revenue (2032) USD 32.9 Bn CAGR (2023-2032) 12.3% Base Year for Estimation 2022 Historic Period 2016-2022 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product Type (Clinical analytics platforms, Electronic data capture, Clinical data management systems, Safety solutions, Randomization & trial supply management, Electronic clinical outcome assessment, Clinical data integration platforms, Clinical trial management systems, Electronic trial master file, Regulatory information management solutions)
By Delivery Mode (Web-hosted, Licensed enterprise, Cloud-based solutions)
By clinical trial (Phase I, Phase II, Phase III, Phase IV)
By End Users (Pharmaceutical, Biopharmaceutical companies, Contract research organizations, Consulting service companies, Medical device manufacturers, Hospitals, Academic research institutions)Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Parexel International Corporation, Oracle Corporation, Merge Health Incorporated, Datatrak International, Inc., BioClinica, CRF Health, ERT, E-Clinical Solutions, OmniComm Systems Inc, Medidata Solution Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
- 1. Executive Summary
- 1.1. Definition
- 1.2. Taxonomy
- 1.3. Research Scope
- 1.4. Key Analysis
- 1.5. Key Findings by Major Segments
- 1.6. Top strategies by Major Players
- 2. Global Eclinical Solutions Market Overview
- 2.1. Eclinical Solutions Market Dynamics
- 2.1.1. Drivers
- 2.1.2. Opportunities
- 2.1.3. Restraints
- 2.1.4. Challenges
- 2.2. Macro-economic Factors
- 2.3. Regulatory Framework
- 2.4. Market Investment Feasibility Index
- 2.5. PEST Analysis
- 2.6. PORTER’S Five Force Analysis
- 2.7. Drivers & Restraints Impact Analysis
- 2.8. Industry Chain Analysis
- 2.9. Cost Structure Analysis
- 2.10. Marketing Strategy
- 2.11. Russia-Ukraine War Impact Analysis
- 2.12. Opportunity Map Analysis
- 2.13. Market Competition Scenario Analysis
- 2.14. Product Life Cycle Analysis
- 2.15. Opportunity Orbits
- 2.16. Manufacturer Intensity Map
- 2.17. Major Companies sales by Value & Volume
- 2.1. Eclinical Solutions Market Dynamics
- 3. Global Eclinical Solutions Market Analysis, Opportunity and Forecast, 2016-2032
- 3.1. Global Eclinical Solutions Market Analysis, 2016-2021
- 3.2. Global Eclinical Solutions Market Opportunity and Forecast, 2023-2032
- 3.3. Global Eclinical Solutions Market Analysis, Opportunity and Forecast, By By Product Type, 2016-2032
- 3.3.1. Global Eclinical Solutions Market Analysis by By Product Type: Introduction
- 3.3.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By Product Type, 2016-2032
- 3.3.3. Clinical analytics platforms
- 3.3.4. Electronic data capture
- 3.3.5. Clinical data management systems
- 3.3.6. Safety solutions
- 3.3.7. Randomization & trial supply management
- 3.3.8. Electronic clinical outcome assessment
- 3.3.9. Clinical data integration platforms
- 3.3.10. Clinical trial management systems
- 3.3.11. Electronic trial master file
- 3.3.12. Regulatory information management solutions
- 3.4. Global Eclinical Solutions Market Analysis, Opportunity and Forecast, By By Delivery Mode, 2016-2032
- 3.4.1. Global Eclinical Solutions Market Analysis by By Delivery Mode: Introduction
- 3.4.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By Delivery Mode, 2016-2032
- 3.4.3. Web-hosted
- 3.4.3.1. Drippers
- 3.4.3.2. Tubing
- 3.4.3.3. Valves and Filters
- 3.4.3.4. Pressure Regulators
- 3.4.3.5. Other
- 3.4.4. Licensed enterprise
- 3.4.4.1. Tubing
- 3.4.4.2. Nozzles
- 3.4.4.3. Pressure Regulators
- 3.4.4.4. Other
- 3.4.5. Cloud-based solutions
- 3.5. Global Eclinical Solutions Market Analysis, Opportunity and Forecast, By By clinical trial, 2016-2032
- 3.5.1. Global Eclinical Solutions Market Analysis by By clinical trial: Introduction
- 3.5.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By clinical trial, 2016-2032
- 3.5.3. Phase I
- 3.5.4. Phase II
- 3.5.5. Phase III
- 3.5.6. Phase IV
- 3.6. Global Eclinical Solutions Market Analysis, Opportunity and Forecast, By By End Users, 2016-2032
- 3.6.1. Global Eclinical Solutions Market Analysis by By End Users: Introduction
- 3.6.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By End Users, 2016-2032
- 3.6.3. Pharmaceutical
- 3.6.4. Biopharmaceutical companies
- 3.6.5. Contract research organizations
- 3.6.6. Consulting service companies
- 3.6.7. Medical device manufacturers
- 3.6.8. Hospitals
- 3.6.9. Academic research institutions
- 4. North America Eclinical Solutions Market Analysis, Opportunity and Forecast, 2016-2032
- 4.1. North America Eclinical Solutions Market Analysis, 2016-2021
- 4.2. North America Eclinical Solutions Market Opportunity and Forecast, 2023-2032
- 4.3. North America Eclinical Solutions Market Analysis, Opportunity and Forecast, By By Product Type, 2016-2032
- 4.3.1. North America Eclinical Solutions Market Analysis by By Product Type: Introduction
- 4.3.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By Product Type, 2016-2032
- 4.3.3. Clinical analytics platforms
- 4.3.4. Electronic data capture
- 4.3.5. Clinical data management systems
- 4.3.6. Safety solutions
- 4.3.7. Randomization & trial supply management
- 4.3.8. Electronic clinical outcome assessment
- 4.3.9. Clinical data integration platforms
- 4.3.10. Clinical trial management systems
- 4.3.11. Electronic trial master file
- 4.3.12. Regulatory information management solutions
- 4.4. North America Eclinical Solutions Market Analysis, Opportunity and Forecast, By By Delivery Mode, 2016-2032
- 4.4.1. North America Eclinical Solutions Market Analysis by By Delivery Mode: Introduction
- 4.4.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By Delivery Mode, 2016-2032
- 4.4.3. Web-hosted
- 4.4.3.1. Drippers
- 4.4.3.2. Tubing
- 4.4.3.3. Valves and Filters
- 4.4.3.4. Pressure Regulators
- 4.4.3.5. Other
- 4.4.4. Licensed enterprise
- 4.4.4.1. Tubing
- 4.4.4.2. Nozzles
- 4.4.4.3. Pressure Regulators
- 4.4.4.4. Other
- 4.4.5. Cloud-based solutions
- 4.5. North America Eclinical Solutions Market Analysis, Opportunity and Forecast, By By clinical trial, 2016-2032
- 4.5.1. North America Eclinical Solutions Market Analysis by By clinical trial: Introduction
- 4.5.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By clinical trial, 2016-2032
- 4.5.3. Phase I
- 4.5.4. Phase II
- 4.5.5. Phase III
- 4.5.6. Phase IV
- 4.6. North America Eclinical Solutions Market Analysis, Opportunity and Forecast, By By End Users, 2016-2032
- 4.6.1. North America Eclinical Solutions Market Analysis by By End Users: Introduction
- 4.6.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By End Users, 2016-2032
- 4.6.3. Pharmaceutical
- 4.6.4. Biopharmaceutical companies
- 4.6.5. Contract research organizations
- 4.6.6. Consulting service companies
- 4.6.7. Medical device manufacturers
- 4.6.8. Hospitals
- 4.6.9. Academic research institutions
- 4.7. North America Eclinical Solutions Market Analysis, Opportunity and Forecast, By Country , 2016-2032
- 4.7.1. North America Eclinical Solutions Market Analysis by Country : Introduction
- 4.7.2. Market Size Absolute $ Opportunity Analysis and Forecast, Country , 2016-2032
- 4.7.2.1. The US
- 4.7.2.2. Canada
- 4.7.2.3. Mexico
- 5. Western Europe Eclinical Solutions Market Analysis, Opportunity and Forecast, 2016-2032
- 5.1. Western Europe Eclinical Solutions Market Analysis, 2016-2021
- 5.2. Western Europe Eclinical Solutions Market Opportunity and Forecast, 2023-2032
- 5.3. Western Europe Eclinical Solutions Market Analysis, Opportunity and Forecast, By By Product Type, 2016-2032
- 5.3.1. Western Europe Eclinical Solutions Market Analysis by By Product Type: Introduction
- 5.3.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By Product Type, 2016-2032
- 5.3.3. Clinical analytics platforms
- 5.3.4. Electronic data capture
- 5.3.5. Clinical data management systems
- 5.3.6. Safety solutions
- 5.3.7. Randomization & trial supply management
- 5.3.8. Electronic clinical outcome assessment
- 5.3.9. Clinical data integration platforms
- 5.3.10. Clinical trial management systems
- 5.3.11. Electronic trial master file
- 5.3.12. Regulatory information management solutions
- 5.4. Western Europe Eclinical Solutions Market Analysis, Opportunity and Forecast, By By Delivery Mode, 2016-2032
- 5.4.1. Western Europe Eclinical Solutions Market Analysis by By Delivery Mode: Introduction
- 5.4.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By Delivery Mode, 2016-2032
- 5.4.3. Web-hosted
- 5.4.3.1. Drippers
- 5.4.3.2. Tubing
- 5.4.3.3. Valves and Filters
- 5.4.3.4. Pressure Regulators
- 5.4.3.5. Other
- 5.4.4. Licensed enterprise
- 5.4.4.1. Tubing
- 5.4.4.2. Nozzles
- 5.4.4.3. Pressure Regulators
- 5.4.4.4. Other
- 5.4.5. Cloud-based solutions
- 5.5. Western Europe Eclinical Solutions Market Analysis, Opportunity and Forecast, By By clinical trial, 2016-2032
- 5.5.1. Western Europe Eclinical Solutions Market Analysis by By clinical trial: Introduction
- 5.5.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By clinical trial, 2016-2032
- 5.5.3. Phase I
- 5.5.4. Phase II
- 5.5.5. Phase III
- 5.5.6. Phase IV
- 5.6. Western Europe Eclinical Solutions Market Analysis, Opportunity and Forecast, By By End Users, 2016-2032
- 5.6.1. Western Europe Eclinical Solutions Market Analysis by By End Users: Introduction
- 5.6.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By End Users, 2016-2032
- 5.6.3. Pharmaceutical
- 5.6.4. Biopharmaceutical companies
- 5.6.5. Contract research organizations
- 5.6.6. Consulting service companies
- 5.6.7. Medical device manufacturers
- 5.6.8. Hospitals
- 5.6.9. Academic research institutions
- 5.7. Western Europe Eclinical Solutions Market Analysis, Opportunity and Forecast, By Country , 2016-2032
- 5.7.1. Western Europe Eclinical Solutions Market Analysis by Country : Introduction
- 5.7.2. Market Size Absolute $ Opportunity Analysis and Forecast, Country , 2016-2032
- 5.7.2.1. Germany
- 5.7.2.2. France
- 5.7.2.3. The UK
- 5.7.2.4. Spain
- 5.7.2.5. Italy
- 5.7.2.6. Portugal
- 5.7.2.7. Ireland
- 5.7.2.8. Austria
- 5.7.2.9. Switzerland
- 5.7.2.10. Benelux
- 5.7.2.11. Nordic
- 5.7.2.12. Rest of Western Europe
- 6. Eastern Europe Eclinical Solutions Market Analysis, Opportunity and Forecast, 2016-2032
- 6.1. Eastern Europe Eclinical Solutions Market Analysis, 2016-2021
- 6.2. Eastern Europe Eclinical Solutions Market Opportunity and Forecast, 2023-2032
- 6.3. Eastern Europe Eclinical Solutions Market Analysis, Opportunity and Forecast, By By Product Type, 2016-2032
- 6.3.1. Eastern Europe Eclinical Solutions Market Analysis by By Product Type: Introduction
- 6.3.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By Product Type, 2016-2032
- 6.3.3. Clinical analytics platforms
- 6.3.4. Electronic data capture
- 6.3.5. Clinical data management systems
- 6.3.6. Safety solutions
- 6.3.7. Randomization & trial supply management
- 6.3.8. Electronic clinical outcome assessment
- 6.3.9. Clinical data integration platforms
- 6.3.10. Clinical trial management systems
- 6.3.11. Electronic trial master file
- 6.3.12. Regulatory information management solutions
- 6.4. Eastern Europe Eclinical Solutions Market Analysis, Opportunity and Forecast, By By Delivery Mode, 2016-2032
- 6.4.1. Eastern Europe Eclinical Solutions Market Analysis by By Delivery Mode: Introduction
- 6.4.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By Delivery Mode, 2016-2032
- 6.4.3. Web-hosted
- 6.4.3.1. Drippers
- 6.4.3.2. Tubing
- 6.4.3.3. Valves and Filters
- 6.4.3.4. Pressure Regulators
- 6.4.3.5. Other
- 6.4.4. Licensed enterprise
- 6.4.4.1. Tubing
- 6.4.4.2. Nozzles
- 6.4.4.3. Pressure Regulators
- 6.4.4.4. Other
- 6.4.5. Cloud-based solutions
- 6.5. Eastern Europe Eclinical Solutions Market Analysis, Opportunity and Forecast, By By clinical trial, 2016-2032
- 6.5.1. Eastern Europe Eclinical Solutions Market Analysis by By clinical trial: Introduction
- 6.5.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By clinical trial, 2016-2032
- 6.5.3. Phase I
- 6.5.4. Phase II
- 6.5.5. Phase III
- 6.5.6. Phase IV
- 6.6. Eastern Europe Eclinical Solutions Market Analysis, Opportunity and Forecast, By By End Users, 2016-2032
- 6.6.1. Eastern Europe Eclinical Solutions Market Analysis by By End Users: Introduction
- 6.6.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By End Users, 2016-2032
- 6.6.3. Pharmaceutical
- 6.6.4. Biopharmaceutical companies
- 6.6.5. Contract research organizations
- 6.6.6. Consulting service companies
- 6.6.7. Medical device manufacturers
- 6.6.8. Hospitals
- 6.6.9. Academic research institutions
- 6.7. Eastern Europe Eclinical Solutions Market Analysis, Opportunity and Forecast, By Country , 2016-2032
- 6.7.1. Eastern Europe Eclinical Solutions Market Analysis by Country : Introduction
- 6.7.2. Market Size Absolute $ Opportunity Analysis and Forecast, Country , 2016-2032
- 6.7.2.1. Russia
- 6.7.2.2. Poland
- 6.7.2.3. The Czech Republic
- 6.7.2.4. Greece
- 6.7.2.5. Rest of Eastern Europe
- 7. APAC Eclinical Solutions Market Analysis, Opportunity and Forecast, 2016-2032
- 7.1. APAC Eclinical Solutions Market Analysis, 2016-2021
- 7.2. APAC Eclinical Solutions Market Opportunity and Forecast, 2023-2032
- 7.3. APAC Eclinical Solutions Market Analysis, Opportunity and Forecast, By By Product Type, 2016-2032
- 7.3.1. APAC Eclinical Solutions Market Analysis by By Product Type: Introduction
- 7.3.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By Product Type, 2016-2032
- 7.3.3. Clinical analytics platforms
- 7.3.4. Electronic data capture
- 7.3.5. Clinical data management systems
- 7.3.6. Safety solutions
- 7.3.7. Randomization & trial supply management
- 7.3.8. Electronic clinical outcome assessment
- 7.3.9. Clinical data integration platforms
- 7.3.10. Clinical trial management systems
- 7.3.11. Electronic trial master file
- 7.3.12. Regulatory information management solutions
- 7.4. APAC Eclinical Solutions Market Analysis, Opportunity and Forecast, By By Delivery Mode, 2016-2032
- 7.4.1. APAC Eclinical Solutions Market Analysis by By Delivery Mode: Introduction
- 7.4.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By Delivery Mode, 2016-2032
- 7.4.3. Web-hosted
- 7.4.3.1. Drippers
- 7.4.3.2. Tubing
- 7.4.3.3. Valves and Filters
- 7.4.3.4. Pressure Regulators
- 7.4.3.5. Other
- 7.4.4. Licensed enterprise
- 7.4.4.1. Tubing
- 7.4.4.2. Nozzles
- 7.4.4.3. Pressure Regulators
- 7.4.4.4. Other
- 7.4.5. Cloud-based solutions
- 7.5. APAC Eclinical Solutions Market Analysis, Opportunity and Forecast, By By clinical trial, 2016-2032
- 7.5.1. APAC Eclinical Solutions Market Analysis by By clinical trial: Introduction
- 7.5.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By clinical trial, 2016-2032
- 7.5.3. Phase I
- 7.5.4. Phase II
- 7.5.5. Phase III
- 7.5.6. Phase IV
- 7.6. APAC Eclinical Solutions Market Analysis, Opportunity and Forecast, By By End Users, 2016-2032
- 7.6.1. APAC Eclinical Solutions Market Analysis by By End Users: Introduction
- 7.6.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By End Users, 2016-2032
- 7.6.3. Pharmaceutical
- 7.6.4. Biopharmaceutical companies
- 7.6.5. Contract research organizations
- 7.6.6. Consulting service companies
- 7.6.7. Medical device manufacturers
- 7.6.8. Hospitals
- 7.6.9. Academic research institutions
- 7.7. APAC Eclinical Solutions Market Analysis, Opportunity and Forecast, By Country , 2016-2032
- 7.7.1. APAC Eclinical Solutions Market Analysis by Country : Introduction
- 7.7.2. Market Size Absolute $ Opportunity Analysis and Forecast, Country , 2016-2032
- 7.7.2.1. China
- 7.7.2.2. Japan
- 7.7.2.3. South Korea
- 7.7.2.4. India
- 7.7.2.5. Australia & New Zeland
- 7.7.2.6. Indonesia
- 7.7.2.7. Malaysia
- 7.7.2.8. Philippines
- 7.7.2.9. Singapore
- 7.7.2.10. Thailand
- 7.7.2.11. Vietnam
- 7.7.2.12. Rest of APAC
- 8. Latin America Eclinical Solutions Market Analysis, Opportunity and Forecast, 2016-2032
- 8.1. Latin America Eclinical Solutions Market Analysis, 2016-2021
- 8.2. Latin America Eclinical Solutions Market Opportunity and Forecast, 2023-2032
- 8.3. Latin America Eclinical Solutions Market Analysis, Opportunity and Forecast, By By Product Type, 2016-2032
- 8.3.1. Latin America Eclinical Solutions Market Analysis by By Product Type: Introduction
- 8.3.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By Product Type, 2016-2032
- 8.3.3. Clinical analytics platforms
- 8.3.4. Electronic data capture
- 8.3.5. Clinical data management systems
- 8.3.6. Safety solutions
- 8.3.7. Randomization & trial supply management
- 8.3.8. Electronic clinical outcome assessment
- 8.3.9. Clinical data integration platforms
- 8.3.10. Clinical trial management systems
- 8.3.11. Electronic trial master file
- 8.3.12. Regulatory information management solutions
- 8.4. Latin America Eclinical Solutions Market Analysis, Opportunity and Forecast, By By Delivery Mode, 2016-2032
- 8.4.1. Latin America Eclinical Solutions Market Analysis by By Delivery Mode: Introduction
- 8.4.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By Delivery Mode, 2016-2032
- 8.4.3. Web-hosted
- 8.4.3.1. Drippers
- 8.4.3.2. Tubing
- 8.4.3.3. Valves and Filters
- 8.4.3.4. Pressure Regulators
- 8.4.3.5. Other
- 8.4.4. Licensed enterprise
- 8.4.4.1. Tubing
- 8.4.4.2. Nozzles
- 8.4.4.3. Pressure Regulators
- 8.4.4.4. Other
- 8.4.5. Cloud-based solutions
- 8.5. Latin America Eclinical Solutions Market Analysis, Opportunity and Forecast, By By clinical trial, 2016-2032
- 8.5.1. Latin America Eclinical Solutions Market Analysis by By clinical trial: Introduction
- 8.5.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By clinical trial, 2016-2032
- 8.5.3. Phase I
- 8.5.4. Phase II
- 8.5.5. Phase III
- 8.5.6. Phase IV
- 8.6. Latin America Eclinical Solutions Market Analysis, Opportunity and Forecast, By By End Users, 2016-2032
- 8.6.1. Latin America Eclinical Solutions Market Analysis by By End Users: Introduction
- 8.6.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By End Users, 2016-2032
- 8.6.3. Pharmaceutical
- 8.6.4. Biopharmaceutical companies
- 8.6.5. Contract research organizations
- 8.6.6. Consulting service companies
- 8.6.7. Medical device manufacturers
- 8.6.8. Hospitals
- 8.6.9. Academic research institutions
- 8.7. Latin America Eclinical Solutions Market Analysis, Opportunity and Forecast, By Country , 2016-2032
- 8.7.1. Latin America Eclinical Solutions Market Analysis by Country : Introduction
- 8.7.2. Market Size Absolute $ Opportunity Analysis and Forecast, Country , 2016-2032
- 8.7.2.1. Brazil
- 8.7.2.2. Colombia
- 8.7.2.3. Chile
- 8.7.2.4. Argentina
- 8.7.2.5. Costa Rica
- 8.7.2.6. Rest of Latin America
- 9. Middle East & Africa Eclinical Solutions Market Analysis, Opportunity and Forecast, 2016-2032
- 9.1. Middle East & Africa Eclinical Solutions Market Analysis, 2016-2021
- 9.2. Middle East & Africa Eclinical Solutions Market Opportunity and Forecast, 2023-2032
- 9.3. Middle East & Africa Eclinical Solutions Market Analysis, Opportunity and Forecast, By By Product Type, 2016-2032
- 9.3.1. Middle East & Africa Eclinical Solutions Market Analysis by By Product Type: Introduction
- 9.3.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By Product Type, 2016-2032
- 9.3.3. Clinical analytics platforms
- 9.3.4. Electronic data capture
- 9.3.5. Clinical data management systems
- 9.3.6. Safety solutions
- 9.3.7. Randomization & trial supply management
- 9.3.8. Electronic clinical outcome assessment
- 9.3.9. Clinical data integration platforms
- 9.3.10. Clinical trial management systems
- 9.3.11. Electronic trial master file
- 9.3.12. Regulatory information management solutions
- 9.4. Middle East & Africa Eclinical Solutions Market Analysis, Opportunity and Forecast, By By Delivery Mode, 2016-2032
- 9.4.1. Middle East & Africa Eclinical Solutions Market Analysis by By Delivery Mode: Introduction
- 9.4.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By Delivery Mode, 2016-2032
- 9.4.3. Web-hosted
- 9.4.3.1. Drippers
- 9.4.3.2. Tubing
- 9.4.3.3. Valves and Filters
- 9.4.3.4. Pressure Regulators
- 9.4.3.5. Other
- 9.4.4. Licensed enterprise
- 9.4.4.1. Tubing
- 9.4.4.2. Nozzles
- 9.4.4.3. Pressure Regulators
- 9.4.4.4. Other
- 9.4.5. Cloud-based solutions
- 9.5. Middle East & Africa Eclinical Solutions Market Analysis, Opportunity and Forecast, By By clinical trial, 2016-2032
- 9.5.1. Middle East & Africa Eclinical Solutions Market Analysis by By clinical trial: Introduction
- 9.5.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By clinical trial, 2016-2032
- 9.5.3. Phase I
- 9.5.4. Phase II
- 9.5.5. Phase III
- 9.5.6. Phase IV
- 9.6. Middle East & Africa Eclinical Solutions Market Analysis, Opportunity and Forecast, By By End Users, 2016-2032
- 9.6.1. Middle East & Africa Eclinical Solutions Market Analysis by By End Users: Introduction
- 9.6.2. Market Size Absolute $ Opportunity Analysis and Forecast, By By End Users, 2016-2032
- 9.6.3. Pharmaceutical
- 9.6.4. Biopharmaceutical companies
- 9.6.5. Contract research organizations
- 9.6.6. Consulting service companies
- 9.6.7. Medical device manufacturers
- 9.6.8. Hospitals
- 9.6.9. Academic research institutions
- 9.7. Middle East & Africa Eclinical Solutions Market Analysis, Opportunity and Forecast, By Country , 2016-2032
- 9.7.1. Middle East & Africa Eclinical Solutions Market Analysis by Country : Introduction
- 9.7.2. Market Size Absolute $ Opportunity Analysis and Forecast, Country , 2016-2032
- 9.7.2.1. Algeria
- 9.7.2.2. Egypt
- 9.7.2.3. Israel
- 9.7.2.4. Kuwait
- 9.7.2.5. Nigeria
- 9.7.2.6. Saudi Arabia
- 9.7.2.7. South Africa
- 9.7.2.8. Turkey
- 9.7.2.9. The UAE
- 9.7.2.10. Rest of MEA
- 10. Global Eclinical Solutions Market Analysis, Opportunity and Forecast, By Region , 2016-2032
- 10.1. Global Eclinical Solutions Market Analysis by Region : Introduction
- 10.2. Market Size Absolute $ Opportunity Analysis and Forecast, By Region , 2016-2032
- 10.2.1. North America
- 10.2.2. Western Europe
- 10.2.3. Eastern Europe
- 10.2.4. APAC
- 10.2.5. Latin America
- 10.2.6. Middle East & Africa
- 11. Global Eclinical Solutions Market Competitive Landscape, Market Share Analysis, and Company Profiles
- 11.1. Market Share Analysis
- 11.2. Company Profiles
- 11.3. Parexel International Corporation
- 11.3.1. Company Overview
- 11.3.2. Financial Highlights
- 11.3.3. Product Portfolio
- 11.3.4. SWOT Analysis
- 11.3.5. Key Strategies and Developments
- 11.4. Oracle Corporation
- 11.4.1. Company Overview
- 11.4.2. Financial Highlights
- 11.4.3. Product Portfolio
- 11.4.4. SWOT Analysis
- 11.4.5. Key Strategies and Developments
- 11.5. Merge Health Incorporated
- 11.5.1. Company Overview
- 11.5.2. Financial Highlights
- 11.5.3. Product Portfolio
- 11.5.4. SWOT Analysis
- 11.5.5. Key Strategies and Developments
- 11.6. Datatrak International, Inc.
- 11.6.1. Company Overview
- 11.6.2. Financial Highlights
- 11.6.3. Product Portfolio
- 11.6.4. SWOT Analysis
- 11.6.5. Key Strategies and Developments
- 11.7. BioClinica
- 11.7.1. Company Overview
- 11.7.2. Financial Highlights
- 11.7.3. Product Portfolio
- 11.7.4. SWOT Analysis
- 11.7.5. Key Strategies and Developments
- 11.8. CRF Health
- 11.8.1. Company Overview
- 11.8.2. Financial Highlights
- 11.8.3. Product Portfolio
- 11.8.4. SWOT Analysis
- 11.8.5. Key Strategies and Developments
- 11.9. ERT
- 11.9.1. Company Overview
- 11.9.2. Financial Highlights
- 11.9.3. Product Portfolio
- 11.9.4. SWOT Analysis
- 11.9.5. Key Strategies and Developments
- 11.10. E-Clinical Solutions
- 11.10.1. Company Overview
- 11.10.2. Financial Highlights
- 11.10.3. Product Portfolio
- 11.10.4. SWOT Analysis
- 11.10.5. Key Strategies and Developments
- 11.11. OmniComm Systems Inc
- 11.11.1. Company Overview
- 11.11.2. Financial Highlights
- 11.11.3. Product Portfolio
- 11.11.4. SWOT Analysis
- 11.11.5. Key Strategies and Developments
- 11.12. Medidata Solution
- 11.12.1. Company Overview
- 11.12.2. Financial Highlights
- 11.12.3. Product Portfolio
- 11.12.4. SWOT Analysis
- 11.12.5. Key Strategies and Developments
- 12. Assumptions and Acronyms
- 13. Research Methodology
- 14. Contact
- List of Figures
- Figure 1: Global Eclinical Solutions Market Revenue (US$ Mn) Market Share by By Product Type in 2022
- Figure 2: Global Eclinical Solutions Market Attractiveness Analysis by By Product Type, 2016-2032
- Figure 3: Global Eclinical Solutions Market Revenue (US$ Mn) Market Share by By Delivery Modein 2022
- Figure 4: Global Eclinical Solutions Market Attractiveness Analysis by By Delivery Mode, 2016-2032
- Figure 5: Global Eclinical Solutions Market Revenue (US$ Mn) Market Share by By clinical trialin 2022
- Figure 6: Global Eclinical Solutions Market Attractiveness Analysis by By clinical trial, 2016-2032
- Figure 7: Global Eclinical Solutions Market Revenue (US$ Mn) Market Share by By End Usersin 2022
- Figure 8: Global Eclinical Solutions Market Attractiveness Analysis by By End Users, 2016-2032
- Figure 9: Global Eclinical Solutions Market Revenue (US$ Mn) Market Share by Region in 2022
- Figure 10: Global Eclinical Solutions Market Attractiveness Analysis by Region, 2016-2032
- Figure 11: Global Eclinical Solutions Market Revenue (US$ Mn) (2016-2032)
- Figure 12: Global Eclinical Solutions Market Revenue (US$ Mn) Comparison by Region (2016-2032)
- Figure 13: Global Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Product Type (2016-2032)
- Figure 14: Global Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Delivery Mode (2016-2032)
- Figure 15: Global Eclinical Solutions Market Revenue (US$ Mn) Comparison by By clinical trial (2016-2032)
- Figure 16: Global Eclinical Solutions Market Revenue (US$ Mn) Comparison by By End Users (2016-2032)
- Figure 17: Global Eclinical Solutions Market Y-o-Y Growth Rate Comparison by Region (2016-2032)
- Figure 18: Global Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Product Type (2016-2032)
- Figure 19: Global Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Delivery Mode (2016-2032)
- Figure 20: Global Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By clinical trial (2016-2032)
- Figure 21: Global Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By End Users (2016-2032)
- Figure 22: Global Eclinical Solutions Market Share Comparison by Region (2016-2032)
- Figure 23: Global Eclinical Solutions Market Share Comparison by By Product Type (2016-2032)
- Figure 24: Global Eclinical Solutions Market Share Comparison by By Delivery Mode (2016-2032)
- Figure 25: Global Eclinical Solutions Market Share Comparison by By clinical trial (2016-2032)
- Figure 26: Global Eclinical Solutions Market Share Comparison by By End Users (2016-2032)
- Figure 27: North America Eclinical Solutions Market Revenue (US$ Mn) Market Share by By Product Typein 2022
- Figure 28: North America Eclinical Solutions Market Attractiveness Analysis by By Product Type, 2016-2032
- Figure 29: North America Eclinical Solutions Market Revenue (US$ Mn) Market Share by By Delivery Modein 2022
- Figure 30: North America Eclinical Solutions Market Attractiveness Analysis by By Delivery Mode, 2016-2032
- Figure 31: North America Eclinical Solutions Market Revenue (US$ Mn) Market Share by By clinical trialin 2022
- Figure 32: North America Eclinical Solutions Market Attractiveness Analysis by By clinical trial, 2016-2032
- Figure 33: North America Eclinical Solutions Market Revenue (US$ Mn) Market Share by By End Usersin 2022
- Figure 34: North America Eclinical Solutions Market Attractiveness Analysis by By End Users, 2016-2032
- Figure 35: North America Eclinical Solutions Market Revenue (US$ Mn) Market Share by Country in 2022
- Figure 36: North America Eclinical Solutions Market Attractiveness Analysis by Country, 2016-2032
- Figure 37: North America Eclinical Solutions Market Revenue (US$ Mn) (2016-2032)
- Figure 38: North America Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Figure 39: North America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Product Type (2016-2032)
- Figure 40: North America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Delivery Mode (2016-2032)
- Figure 41: North America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By clinical trial (2016-2032)
- Figure 42: North America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By End Users (2016-2032)
- Figure 43: North America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by Country (2016-2032)
- Figure 44: North America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Product Type (2016-2032)
- Figure 45: North America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Delivery Mode (2016-2032)
- Figure 46: North America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By clinical trial (2016-2032)
- Figure 47: North America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By End Users (2016-2032)
- Figure 48: North America Eclinical Solutions Market Share Comparison by Country (2016-2032)
- Figure 49: North America Eclinical Solutions Market Share Comparison by By Product Type (2016-2032)
- Figure 50: North America Eclinical Solutions Market Share Comparison by By Delivery Mode (2016-2032)
- Figure 51: North America Eclinical Solutions Market Share Comparison by By clinical trial (2016-2032)
- Figure 52: North America Eclinical Solutions Market Share Comparison by By End Users (2016-2032)
- Figure 53: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Market Share by By Product Typein 2022
- Figure 54: Western Europe Eclinical Solutions Market Attractiveness Analysis by By Product Type, 2016-2032
- Figure 55: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Market Share by By Delivery Modein 2022
- Figure 56: Western Europe Eclinical Solutions Market Attractiveness Analysis by By Delivery Mode, 2016-2032
- Figure 57: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Market Share by By clinical trialin 2022
- Figure 58: Western Europe Eclinical Solutions Market Attractiveness Analysis by By clinical trial, 2016-2032
- Figure 59: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Market Share by By End Usersin 2022
- Figure 60: Western Europe Eclinical Solutions Market Attractiveness Analysis by By End Users, 2016-2032
- Figure 61: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Market Share by Country in 2022
- Figure 62: Western Europe Eclinical Solutions Market Attractiveness Analysis by Country, 2016-2032
- Figure 63: Western Europe Eclinical Solutions Market Revenue (US$ Mn) (2016-2032)
- Figure 64: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Figure 65: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Product Type (2016-2032)
- Figure 66: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Delivery Mode (2016-2032)
- Figure 67: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By clinical trial (2016-2032)
- Figure 68: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By End Users (2016-2032)
- Figure 69: Western Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by Country (2016-2032)
- Figure 70: Western Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Product Type (2016-2032)
- Figure 71: Western Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Delivery Mode (2016-2032)
- Figure 72: Western Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By clinical trial (2016-2032)
- Figure 73: Western Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By End Users (2016-2032)
- Figure 74: Western Europe Eclinical Solutions Market Share Comparison by Country (2016-2032)
- Figure 75: Western Europe Eclinical Solutions Market Share Comparison by By Product Type (2016-2032)
- Figure 76: Western Europe Eclinical Solutions Market Share Comparison by By Delivery Mode (2016-2032)
- Figure 77: Western Europe Eclinical Solutions Market Share Comparison by By clinical trial (2016-2032)
- Figure 78: Western Europe Eclinical Solutions Market Share Comparison by By End Users (2016-2032)
- Figure 79: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Market Share by By Product Typein 2022
- Figure 80: Eastern Europe Eclinical Solutions Market Attractiveness Analysis by By Product Type, 2016-2032
- Figure 81: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Market Share by By Delivery Modein 2022
- Figure 82: Eastern Europe Eclinical Solutions Market Attractiveness Analysis by By Delivery Mode, 2016-2032
- Figure 83: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Market Share by By clinical trialin 2022
- Figure 84: Eastern Europe Eclinical Solutions Market Attractiveness Analysis by By clinical trial, 2016-2032
- Figure 85: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Market Share by By End Usersin 2022
- Figure 86: Eastern Europe Eclinical Solutions Market Attractiveness Analysis by By End Users, 2016-2032
- Figure 87: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Market Share by Country in 2022
- Figure 88: Eastern Europe Eclinical Solutions Market Attractiveness Analysis by Country, 2016-2032
- Figure 89: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) (2016-2032)
- Figure 90: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Figure 91: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Product Type (2016-2032)
- Figure 92: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Delivery Mode (2016-2032)
- Figure 93: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By clinical trial (2016-2032)
- Figure 94: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By End Users (2016-2032)
- Figure 95: Eastern Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by Country (2016-2032)
- Figure 96: Eastern Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Product Type (2016-2032)
- Figure 97: Eastern Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Delivery Mode (2016-2032)
- Figure 98: Eastern Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By clinical trial (2016-2032)
- Figure 99: Eastern Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By End Users (2016-2032)
- Figure 100: Eastern Europe Eclinical Solutions Market Share Comparison by Country (2016-2032)
- Figure 101: Eastern Europe Eclinical Solutions Market Share Comparison by By Product Type (2016-2032)
- Figure 102: Eastern Europe Eclinical Solutions Market Share Comparison by By Delivery Mode (2016-2032)
- Figure 103: Eastern Europe Eclinical Solutions Market Share Comparison by By clinical trial (2016-2032)
- Figure 104: Eastern Europe Eclinical Solutions Market Share Comparison by By End Users (2016-2032)
- Figure 105: APAC Eclinical Solutions Market Revenue (US$ Mn) Market Share by By Product Typein 2022
- Figure 106: APAC Eclinical Solutions Market Attractiveness Analysis by By Product Type, 2016-2032
- Figure 107: APAC Eclinical Solutions Market Revenue (US$ Mn) Market Share by By Delivery Modein 2022
- Figure 108: APAC Eclinical Solutions Market Attractiveness Analysis by By Delivery Mode, 2016-2032
- Figure 109: APAC Eclinical Solutions Market Revenue (US$ Mn) Market Share by By clinical trialin 2022
- Figure 110: APAC Eclinical Solutions Market Attractiveness Analysis by By clinical trial, 2016-2032
- Figure 111: APAC Eclinical Solutions Market Revenue (US$ Mn) Market Share by By End Usersin 2022
- Figure 112: APAC Eclinical Solutions Market Attractiveness Analysis by By End Users, 2016-2032
- Figure 113: APAC Eclinical Solutions Market Revenue (US$ Mn) Market Share by Country in 2022
- Figure 114: APAC Eclinical Solutions Market Attractiveness Analysis by Country, 2016-2032
- Figure 115: APAC Eclinical Solutions Market Revenue (US$ Mn) (2016-2032)
- Figure 116: APAC Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Figure 117: APAC Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Product Type (2016-2032)
- Figure 118: APAC Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Delivery Mode (2016-2032)
- Figure 119: APAC Eclinical Solutions Market Revenue (US$ Mn) Comparison by By clinical trial (2016-2032)
- Figure 120: APAC Eclinical Solutions Market Revenue (US$ Mn) Comparison by By End Users (2016-2032)
- Figure 121: APAC Eclinical Solutions Market Y-o-Y Growth Rate Comparison by Country (2016-2032)
- Figure 122: APAC Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Product Type (2016-2032)
- Figure 123: APAC Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Delivery Mode (2016-2032)
- Figure 124: APAC Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By clinical trial (2016-2032)
- Figure 125: APAC Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By End Users (2016-2032)
- Figure 126: APAC Eclinical Solutions Market Share Comparison by Country (2016-2032)
- Figure 127: APAC Eclinical Solutions Market Share Comparison by By Product Type (2016-2032)
- Figure 128: APAC Eclinical Solutions Market Share Comparison by By Delivery Mode (2016-2032)
- Figure 129: APAC Eclinical Solutions Market Share Comparison by By clinical trial (2016-2032)
- Figure 130: APAC Eclinical Solutions Market Share Comparison by By End Users (2016-2032)
- Figure 131: Latin America Eclinical Solutions Market Revenue (US$ Mn) Market Share by By Product Typein 2022
- Figure 132: Latin America Eclinical Solutions Market Attractiveness Analysis by By Product Type, 2016-2032
- Figure 133: Latin America Eclinical Solutions Market Revenue (US$ Mn) Market Share by By Delivery Modein 2022
- Figure 134: Latin America Eclinical Solutions Market Attractiveness Analysis by By Delivery Mode, 2016-2032
- Figure 135: Latin America Eclinical Solutions Market Revenue (US$ Mn) Market Share by By clinical trialin 2022
- Figure 136: Latin America Eclinical Solutions Market Attractiveness Analysis by By clinical trial, 2016-2032
- Figure 137: Latin America Eclinical Solutions Market Revenue (US$ Mn) Market Share by By End Usersin 2022
- Figure 138: Latin America Eclinical Solutions Market Attractiveness Analysis by By End Users, 2016-2032
- Figure 139: Latin America Eclinical Solutions Market Revenue (US$ Mn) Market Share by Country in 2022
- Figure 140: Latin America Eclinical Solutions Market Attractiveness Analysis by Country, 2016-2032
- Figure 141: Latin America Eclinical Solutions Market Revenue (US$ Mn) (2016-2032)
- Figure 142: Latin America Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Figure 143: Latin America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Product Type (2016-2032)
- Figure 144: Latin America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Delivery Mode (2016-2032)
- Figure 145: Latin America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By clinical trial (2016-2032)
- Figure 146: Latin America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By End Users (2016-2032)
- Figure 147: Latin America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by Country (2016-2032)
- Figure 148: Latin America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Product Type (2016-2032)
- Figure 149: Latin America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Delivery Mode (2016-2032)
- Figure 150: Latin America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By clinical trial (2016-2032)
- Figure 151: Latin America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By End Users (2016-2032)
- Figure 152: Latin America Eclinical Solutions Market Share Comparison by Country (2016-2032)
- Figure 153: Latin America Eclinical Solutions Market Share Comparison by By Product Type (2016-2032)
- Figure 154: Latin America Eclinical Solutions Market Share Comparison by By Delivery Mode (2016-2032)
- Figure 155: Latin America Eclinical Solutions Market Share Comparison by By clinical trial (2016-2032)
- Figure 156: Latin America Eclinical Solutions Market Share Comparison by By End Users (2016-2032)
- Figure 157: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Market Share by By Product Typein 2022
- Figure 158: Middle East & Africa Eclinical Solutions Market Attractiveness Analysis by By Product Type, 2016-2032
- Figure 159: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Market Share by By Delivery Modein 2022
- Figure 160: Middle East & Africa Eclinical Solutions Market Attractiveness Analysis by By Delivery Mode, 2016-2032
- Figure 161: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Market Share by By clinical trialin 2022
- Figure 162: Middle East & Africa Eclinical Solutions Market Attractiveness Analysis by By clinical trial, 2016-2032
- Figure 163: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Market Share by By End Usersin 2022
- Figure 164: Middle East & Africa Eclinical Solutions Market Attractiveness Analysis by By End Users, 2016-2032
- Figure 165: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Market Share by Country in 2022
- Figure 166: Middle East & Africa Eclinical Solutions Market Attractiveness Analysis by Country, 2016-2032
- Figure 167: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) (2016-2032)
- Figure 168: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Figure 169: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Product Type (2016-2032)
- Figure 170: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Delivery Mode (2016-2032)
- Figure 171: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Comparison by By clinical trial (2016-2032)
- Figure 172: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Comparison by By End Users (2016-2032)
- Figure 173: Middle East & Africa Eclinical Solutions Market Y-o-Y Growth Rate Comparison by Country (2016-2032)
- Figure 174: Middle East & Africa Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Product Type (2016-2032)
- Figure 175: Middle East & Africa Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Delivery Mode (2016-2032)
- Figure 176: Middle East & Africa Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By clinical trial (2016-2032)
- Figure 177: Middle East & Africa Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By End Users (2016-2032)
- Figure 178: Middle East & Africa Eclinical Solutions Market Share Comparison by Country (2016-2032)
- Figure 179: Middle East & Africa Eclinical Solutions Market Share Comparison by By Product Type (2016-2032)
- Figure 180: Middle East & Africa Eclinical Solutions Market Share Comparison by By Delivery Mode (2016-2032)
- Figure 181: Middle East & Africa Eclinical Solutions Market Share Comparison by By clinical trial (2016-2032)
- Figure 182: Middle East & Africa Eclinical Solutions Market Share Comparison by By End Users (2016-2032)
- List of Tables
- Table 1: Global Eclinical Solutions Market Comparison by By Product Type (2016-2032)
- Table 2: Global Eclinical Solutions Market Comparison by By Delivery Mode (2016-2032)
- Table 3: Global Eclinical Solutions Market Comparison by By clinical trial (2016-2032)
- Table 4: Global Eclinical Solutions Market Comparison by By End Users (2016-2032)
- Table 5: Global Eclinical Solutions Market Revenue (US$ Mn) Comparison by Region (2016-2032)
- Table 6: Global Eclinical Solutions Market Revenue (US$ Mn) (2016-2032)
- Table 7: Global Eclinical Solutions Market Revenue (US$ Mn) Comparison by Region (2016-2032)
- Table 8: Global Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Product Type (2016-2032)
- Table 9: Global Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Delivery Mode (2016-2032)
- Table 10: Global Eclinical Solutions Market Revenue (US$ Mn) Comparison by By clinical trial (2016-2032)
- Table 11: Global Eclinical Solutions Market Revenue (US$ Mn) Comparison by By End Users (2016-2032)
- Table 12: Global Eclinical Solutions Market Y-o-Y Growth Rate Comparison by Region (2016-2032)
- Table 13: Global Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Product Type (2016-2032)
- Table 14: Global Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Delivery Mode (2016-2032)
- Table 15: Global Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By clinical trial (2016-2032)
- Table 16: Global Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By End Users (2016-2032)
- Table 17: Global Eclinical Solutions Market Share Comparison by Region (2016-2032)
- Table 18: Global Eclinical Solutions Market Share Comparison by By Product Type (2016-2032)
- Table 19: Global Eclinical Solutions Market Share Comparison by By Delivery Mode (2016-2032)
- Table 20: Global Eclinical Solutions Market Share Comparison by By clinical trial (2016-2032)
- Table 21: Global Eclinical Solutions Market Share Comparison by By End Users (2016-2032)
- Table 22: North America Eclinical Solutions Market Comparison by By Delivery Mode (2016-2032)
- Table 23: North America Eclinical Solutions Market Comparison by By clinical trial (2016-2032)
- Table 24: North America Eclinical Solutions Market Comparison by By End Users (2016-2032)
- Table 25: North America Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Table 26: North America Eclinical Solutions Market Revenue (US$ Mn) (2016-2032)
- Table 27: North America Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Table 28: North America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Product Type (2016-2032)
- Table 29: North America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Delivery Mode (2016-2032)
- Table 30: North America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By clinical trial (2016-2032)
- Table 31: North America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By End Users (2016-2032)
- Table 32: North America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by Country (2016-2032)
- Table 33: North America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Product Type (2016-2032)
- Table 34: North America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Delivery Mode (2016-2032)
- Table 35: North America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By clinical trial (2016-2032)
- Table 36: North America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By End Users (2016-2032)
- Table 37: North America Eclinical Solutions Market Share Comparison by Country (2016-2032)
- Table 38: North America Eclinical Solutions Market Share Comparison by By Product Type (2016-2032)
- Table 39: North America Eclinical Solutions Market Share Comparison by By Delivery Mode (2016-2032)
- Table 40: North America Eclinical Solutions Market Share Comparison by By clinical trial (2016-2032)
- Table 41: North America Eclinical Solutions Market Share Comparison by By End Users (2016-2032)
- Table 42: Western Europe Eclinical Solutions Market Comparison by By Product Type (2016-2032)
- Table 43: Western Europe Eclinical Solutions Market Comparison by By Delivery Mode (2016-2032)
- Table 44: Western Europe Eclinical Solutions Market Comparison by By clinical trial (2016-2032)
- Table 45: Western Europe Eclinical Solutions Market Comparison by By End Users (2016-2032)
- Table 46: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Table 47: Western Europe Eclinical Solutions Market Revenue (US$ Mn) (2016-2032)
- Table 48: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Table 49: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Product Type (2016-2032)
- Table 50: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Delivery Mode (2016-2032)
- Table 51: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By clinical trial (2016-2032)
- Table 52: Western Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By End Users (2016-2032)
- Table 53: Western Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by Country (2016-2032)
- Table 54: Western Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Product Type (2016-2032)
- Table 55: Western Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Delivery Mode (2016-2032)
- Table 56: Western Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By clinical trial (2016-2032)
- Table 57: Western Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By End Users (2016-2032)
- Table 58: Western Europe Eclinical Solutions Market Share Comparison by Country (2016-2032)
- Table 59: Western Europe Eclinical Solutions Market Share Comparison by By Product Type (2016-2032)
- Table 60: Western Europe Eclinical Solutions Market Share Comparison by By Delivery Mode (2016-2032)
- Table 61: Western Europe Eclinical Solutions Market Share Comparison by By clinical trial (2016-2032)
- Table 62: Western Europe Eclinical Solutions Market Share Comparison by By End Users (2016-2032)
- Table 63: Eastern Europe Eclinical Solutions Market Comparison by By Product Type (2016-2032)
- Table 64: Eastern Europe Eclinical Solutions Market Comparison by By Delivery Mode (2016-2032)
- Table 65: Eastern Europe Eclinical Solutions Market Comparison by By clinical trial (2016-2032)
- Table 66: Eastern Europe Eclinical Solutions Market Comparison by By End Users (2016-2032)
- Table 67: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Table 68: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) (2016-2032)
- Table 69: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Table 70: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Product Type (2016-2032)
- Table 71: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Delivery Mode (2016-2032)
- Table 72: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By clinical trial (2016-2032)
- Table 73: Eastern Europe Eclinical Solutions Market Revenue (US$ Mn) Comparison by By End Users (2016-2032)
- Table 74: Eastern Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by Country (2016-2032)
- Table 75: Eastern Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Product Type (2016-2032)
- Table 76: Eastern Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Delivery Mode (2016-2032)
- Table 77: Eastern Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By clinical trial (2016-2032)
- Table 78: Eastern Europe Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By End Users (2016-2032)
- Table 79: Eastern Europe Eclinical Solutions Market Share Comparison by Country (2016-2032)
- Table 80: Eastern Europe Eclinical Solutions Market Share Comparison by By Product Type (2016-2032)
- Table 81: Eastern Europe Eclinical Solutions Market Share Comparison by By Delivery Mode (2016-2032)
- Table 82: Eastern Europe Eclinical Solutions Market Share Comparison by By clinical trial (2016-2032)
- Table 83: Eastern Europe Eclinical Solutions Market Share Comparison by By End Users (2016-2032)
- Table 84: APAC Eclinical Solutions Market Comparison by By Product Type (2016-2032)
- Table 85: APAC Eclinical Solutions Market Comparison by By Delivery Mode (2016-2032)
- Table 86: APAC Eclinical Solutions Market Comparison by By clinical trial (2016-2032)
- Table 87: APAC Eclinical Solutions Market Comparison by By End Users (2016-2032)
- Table 88: APAC Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Table 89: APAC Eclinical Solutions Market Revenue (US$ Mn) (2016-2032)
- Table 90: APAC Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Table 91: APAC Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Product Type (2016-2032)
- Table 92: APAC Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Delivery Mode (2016-2032)
- Table 93: APAC Eclinical Solutions Market Revenue (US$ Mn) Comparison by By clinical trial (2016-2032)
- Table 94: APAC Eclinical Solutions Market Revenue (US$ Mn) Comparison by By End Users (2016-2032)
- Table 95: APAC Eclinical Solutions Market Y-o-Y Growth Rate Comparison by Country (2016-2032)
- Table 96: APAC Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Product Type (2016-2032)
- Table 97: APAC Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Delivery Mode (2016-2032)
- Table 98: APAC Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By clinical trial (2016-2032)
- Table 99: APAC Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By End Users (2016-2032)
- Table 100: APAC Eclinical Solutions Market Share Comparison by Country (2016-2032)
- Table 101: APAC Eclinical Solutions Market Share Comparison by By Product Type (2016-2032)
- Table 102: APAC Eclinical Solutions Market Share Comparison by By Delivery Mode (2016-2032)
- Table 103: APAC Eclinical Solutions Market Share Comparison by By clinical trial (2016-2032)
- Table 104: APAC Eclinical Solutions Market Share Comparison by By End Users (2016-2032)
- Table 105: Latin America Eclinical Solutions Market Comparison by By Product Type (2016-2032)
- Table 106: Latin America Eclinical Solutions Market Comparison by By Delivery Mode (2016-2032)
- Table 107: Latin America Eclinical Solutions Market Comparison by By clinical trial (2016-2032)
- Table 108: Latin America Eclinical Solutions Market Comparison by By End Users (2016-2032)
- Table 109: Latin America Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Table 110: Latin America Eclinical Solutions Market Revenue (US$ Mn) (2016-2032)
- Table 111: Latin America Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Table 112: Latin America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Product Type (2016-2032)
- Table 113: Latin America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Delivery Mode (2016-2032)
- Table 114: Latin America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By clinical trial (2016-2032)
- Table 115: Latin America Eclinical Solutions Market Revenue (US$ Mn) Comparison by By End Users (2016-2032)
- Table 116: Latin America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by Country (2016-2032)
- Table 117: Latin America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Product Type (2016-2032)
- Table 118: Latin America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Delivery Mode (2016-2032)
- Table 119: Latin America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By clinical trial (2016-2032)
- Table 120: Latin America Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By End Users (2016-2032)
- Table 121: Latin America Eclinical Solutions Market Share Comparison by Country (2016-2032)
- Table 122: Latin America Eclinical Solutions Market Share Comparison by By Product Type (2016-2032)
- Table 123: Latin America Eclinical Solutions Market Share Comparison by By Delivery Mode (2016-2032)
- Table 124: Latin America Eclinical Solutions Market Share Comparison by By clinical trial (2016-2032)
- Table 125: Latin America Eclinical Solutions Market Share Comparison by By End Users (2016-2032)
- Table 126: Middle East & Africa Eclinical Solutions Market Comparison by By Product Type (2016-2032)
- Table 127: Middle East & Africa Eclinical Solutions Market Comparison by By Delivery Mode (2016-2032)
- Table 128: Middle East & Africa Eclinical Solutions Market Comparison by By clinical trial (2016-2032)
- Table 129: Middle East & Africa Eclinical Solutions Market Comparison by By End Users (2016-2032)
- Table 130: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Table 131: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) (2016-2032)
- Table 132: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Comparison by Country (2016-2032)
- Table 133: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Product Type (2016-2032)
- Table 134: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Comparison by By Delivery Mode (2016-2032)
- Table 135: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Comparison by By clinical trial (2016-2032)
- Table 136: Middle East & Africa Eclinical Solutions Market Revenue (US$ Mn) Comparison by By End Users (2016-2032)
- Table 137: Middle East & Africa Eclinical Solutions Market Y-o-Y Growth Rate Comparison by Country (2016-2032)
- Table 138: Middle East & Africa Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Product Type (2016-2032)
- Table 139: Middle East & Africa Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By Delivery Mode (2016-2032)
- Table 140: Middle East & Africa Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By clinical trial (2016-2032)
- Table 141: Middle East & Africa Eclinical Solutions Market Y-o-Y Growth Rate Comparison by By End Users (2016-2032)
- Table 142: Middle East & Africa Eclinical Solutions Market Share Comparison by Country (2016-2032)
- Table 143: Middle East & Africa Eclinical Solutions Market Share Comparison by By Product Type (2016-2032)
- Table 144: Middle East & Africa Eclinical Solutions Market Share Comparison by By Delivery Mode (2016-2032)
- Table 145: Middle East & Africa Eclinical Solutions Market Share Comparison by By clinical trial (2016-2032)
- Table 146: Middle East & Africa Eclinical Solutions Market Share Comparison by By End Users (2016-2032)
- 1. Executive Summary
-
- Parexel International Corporation
- Oracle Corporation
- Merge Health Incorporated
- Datatrak International, Inc.
- BioClinica
- CRF Health
- ERT
- E-Clinical Solutions
- OmniComm Systems Inc
- Medidata Solution




