
eClinical Solutions Market By Product Type (Electronic data capture, Clinical data management systems, and Others), By Delivery Mode (Web-hosted, Licensed enterprise, Cloud-based solutions), By clinical trial (Phase I, Phase II, and Other), By Region And Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, And Forecast 2023-2032
-
562
-
May 2023
-
163
-
-
This report was compiled by Correspondence Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Report Overview
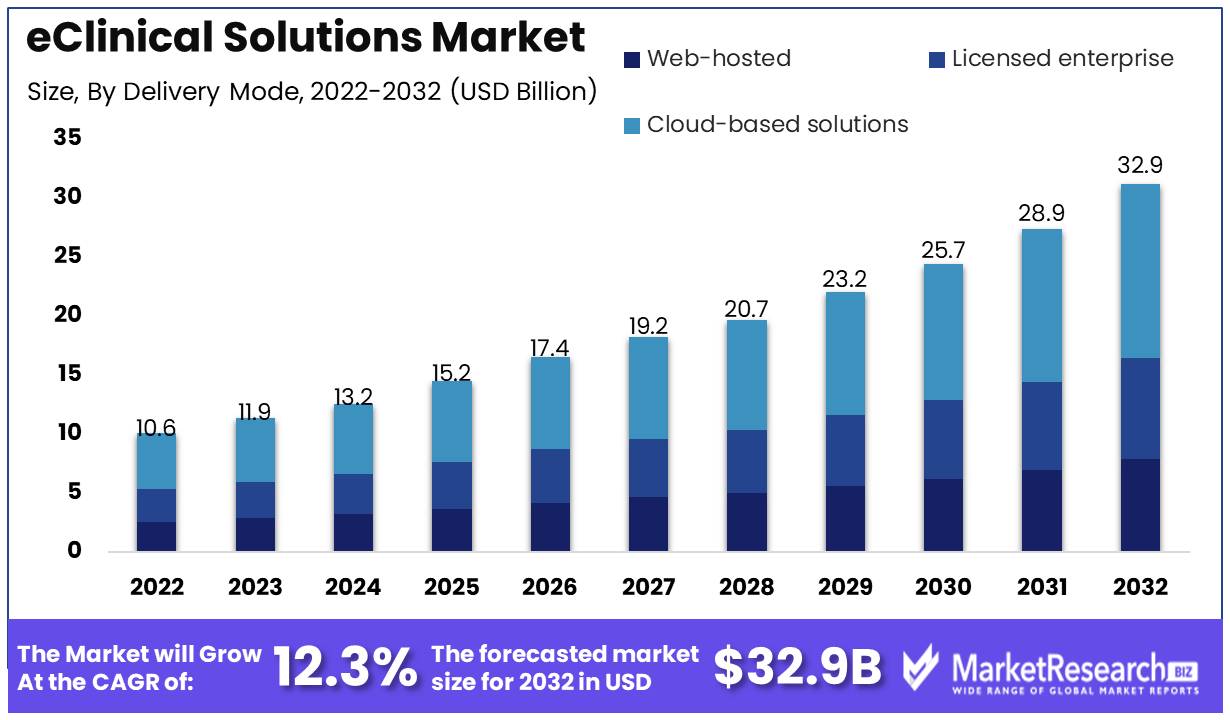
eClinical Solutions Market size is expected to be worth around USD 32.9 Bn by 2032 from USD 10.6 Bn in 2022, growing at a CAGR of 12.3% during the forecast period from 2023 to 2032.
The eClinical Solutions Market has experienced a rapid expansion as numerous industries have invested in this innovative technology. eClinical Solutions incorporate the use of electronic tools and technology to improve the efficacy and precision of clinical trials, data management, and statistical analysis. In this article, we will discuss the eClinical Solutions Market's definition, objectives, significance, advantages, notable innovations, significant investments, and incorporation into products and services. We will examine the expansion and applications of the eClinical solutions market in forecast period.
eClinical Solutions entail the application of electronic tools, medical device and technology to enhance the effectiveness and precision of clinical trials, data management, and analysis. These solutions consist of electronic data capture (EDC), clinical data management systems (CDMS), electronic patient-reported outcomes (ePRO), randomization and trial supply management (RTSM), and clinical trial management systems (CTMS).
The primary objective of eClinical solutions is to improve the efficacy of clinical trials by streamlining data acquisition and reducing the time and costs associated with conducting research. These solutions allow clinical researchers to spend less time on data collection and analysis, so they can devote more time to scientific research and analysis.
E-Clinical Solution are significant due to their numerous advantages over conventional research methods. They increase the speed and efficacy of data collection and analysis, reduce the risk of errors, improve the accuracy and validity of data, and provide real-time access to data, thus accelerating research outcomes.
With the healthcare sector embracing the digital age, e-clinical solutions are at the forefront of this revolution, contributing significantly to the sector's financial success. eCOA, represents a revolutionary shift in patient-reported outcomes. Traditionally, patients would record their experiences on paper, leading to delays and potential data loss. eCOA platforms digitize this process, allowing patients to report their symptoms, side effects, and quality of life directly through user-friendly interfaces.
Data privacy and security is a concern associated with e-clinical solutions from an ethical standpoint. To protect the confidentiality, administration, and sharing of data for both research participants and investigators, it is essential to implement data management and security policies. Ensuring patient safety is paramount in the market. Innovative safety solutions, such as real-time monitoring and adverse event reporting, are gaining prominence to minimize risks associated with these devices.
Driving factors
Streamlining Patient Recruitment and Information Access
The market size for eClinical Solutions has been a game-changer in clinical research studies. Patient recruitment and retention have always been significant obstacles in clinical trials, which can affect the duration and cost of the study. Nonetheless, with the implementation of eClinical solutions, researchers can streamline patient enrollment and increase retention. In clinical research, manual data generation and lack of real-time data access have also been significant pain points. However, eClinical solutions provide real-time data access, making the process more efficient and cost-effective. Real-time data access is critical in healthcare settings, particularly in patient monitoring and medical device management. Healthcare professionals can receive real-time updates on patients' vital signs, ensuring immediate intervention if necessary.
Enhanced Productivity and Cost Effectiveness
Researchers increasingly favor eClinical solutions for their increased efficacy and cost effectiveness. These solutions facilitate the research process and improve site performance. These solutions are being adopted by Medical Device Manufacturers, pharmaceutical companies and independent researchers in order to eliminate procedural bottlenecks and streamline the clinical trial process. CROs and biotech companies have significantly increased their adoption of eClinical solutions in recent years.
Compliance Assistance for eClinical Solutions
Regulatory alterations affecting the market for eClinical Solutions are always possible. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are currently encouraging the adoption of eClinical solutions and streamlining the approval process. These modifications may have a positive effect on the market in forecast period.
Source refers to the point where patient data originates. It serves as the foundation of clinical research. eClinical Solutions leverage Source data integration to ensure data accuracy and consistency. By capturing patient data directly at the source, such as electronic health records (EHRs) and medical devices, researchers eliminate transcription errors and ensure real-time access to critical information.
Restraining Factors
High Costs of Implementation
The high cost of implementation is one of the major restraining factors for eClinical solutions. Typically, the implementation of eClinical solutions requires a substantial investment in software licenses, hardware, and IT support services. Many government and healthcare organizations, particularly modest businesses, find it difficult to designate the resources required to implement these solutions. The high implementation costs are also a major factor that prevents new competitors from entering the market.
Price of Technical Support for Cloud-Based Applications
The price of technical support for cloud-based solutions is an additional cost factor. Due to their adaptability and scalability, cloud-based solutions have grown in popularity, making them an attractive option for many government and healthcare organizations. However, cloud-based solutions require technical expertise, which can be costly to obtain. Organizations that lack the resources to implement cloud-based solutions are compelled to implement costly on-premise alternatives. In such instances, the cost of technical support may pose a substantial barrier to the adoption of cloud-based solutions.
Product Type Analysis
Electronic Data Capture (EDC) dominate eClinical Solutions market segment by products type. With the development of technological advancements, the market for eClinical solutions has been revolutionized, and electronic data capture (EDC) is one of the market's dominant segments. EDC is the process of electronically accumulating clinical trial data, which includes data management, analysis, and presentation. The pharmaceutical and biotechnology industries have employed Electronic Data Capture for the efficient administration of clinical trials. Electronic Data Capture solutions are playing a pivotal role in urology clinical trials by streamlining data collection and management. These tools enhance efficiency, reduce errors, and accelerate the product development by providing real-time access to patient data.
Electronic Data Capture's cost-effectiveness, time-saving, accuracy, and data quality have driven its adoption. Due to its real-time data monitoring and administration, the Electronic Data Capture category will dominate eClinical solutions. Electronic Data Capture can help pharmaceutical and biotechnology companies manage and process enormous amounts of data in real time to improve clinical trial efficiency. Consumers are adopting new healthcare technology. Electronic Data Capture is a popular strategy for clinical trial data collection and management. Electronic Data Capture's accuracy and data quality have encouraged customer acceptance. Consumers favor Electronic Data Capture because to its cost-effectiveness, convenience of access, and data management.
Delivery Mode Analysis
The segment of cloud-based eClinical solutions dominates the eClinical solutions market. One of the leading market segments in the eClinical solutions is Cloud based eClinical solutions. It provides a scalable and secure platform for centrally managing clinical trial data. The cloud-based delivery mode offers a number of advantages, including cost-effectiveness, security, and reduced infrastructure needs. Clinical trial management software is extensively used in the pharmaceutical and biotechnology industries.
Cloud-based eClinical solutions are popular because of their cost-effectiveness, scalability, and accessibility. Consumers choose cloud-based eClinical solutions for clinical trial data management. Consumers choose these solutions because to their better data quality and faster processing. Due to many factors, cloud-based eClinical solutions are expected to develop the quickest. This segment is growing due to the healthcare sector's adoption of digital technologies, the pharmaceutical and biotechnology industries' adoption of cloud-based solutions, and emerging economies' economic growth. Cloud-based eClinical solutions manage clinical trial data cost-effectively, securely, and scalablely.
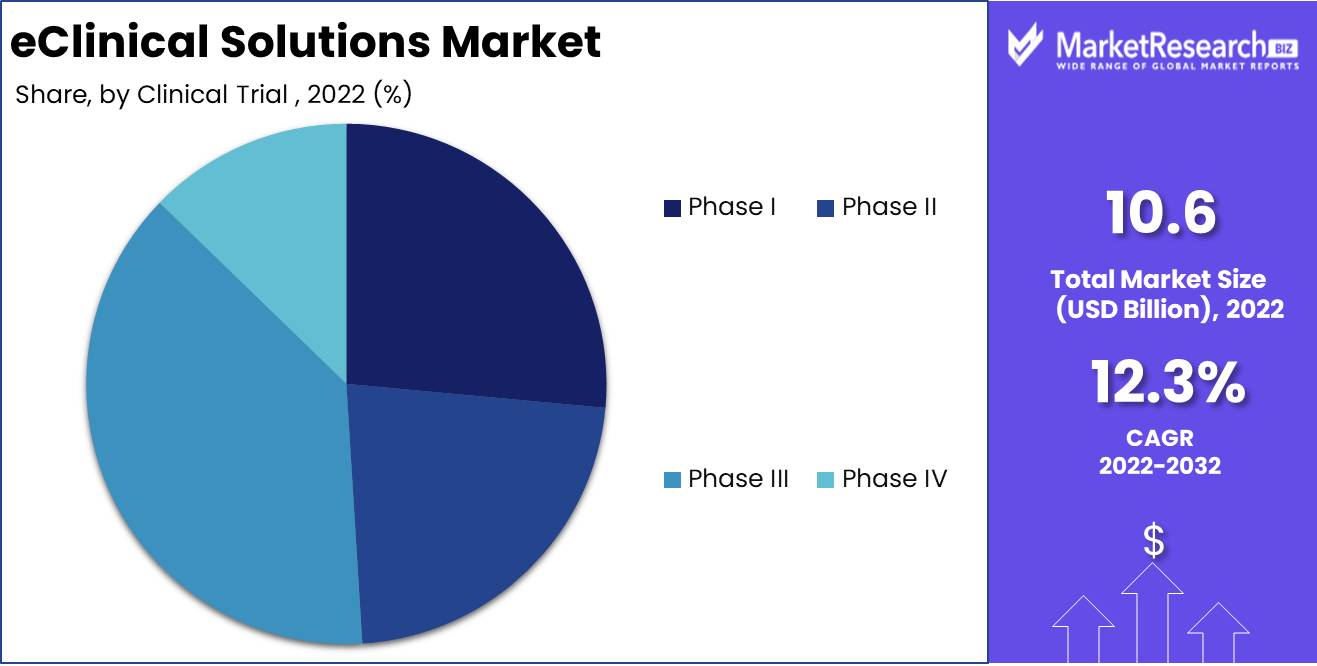
Clinical Trial Phase Analysis
Marked eClinical Solutions Segment Dominates Phase III Clinical Trials. The Clinical Trial Phase III clinical trials segment dominates the market for eClinical solutions. The final and most crucial phase of clinical trials is Clinical Trial Phase III. The success of these trials determines whether regulatory authorities will approve a novel drug or therapy. eClinical solutions are utilized by the pharmaceutical and biotechnology industries for the administration of Clinical Trial Phase III clinical trials due to their cost-effectiveness, quicker data processing, and enhanced data quality.
Consumers like cutting-edge healthcare technology. Due to their cost-effectiveness, speed, and data quality, e-clinical solutions are commonly used to manage Clinical Trial Phase III clinical studies. Consumers prefer these solutions over manual, error-prone clinical trial management. Due to various factors, Phase III clinical trials are expected to increase the fastest. This segment is growing due to the healthcare sector's adoption of digital technologies, the pharmaceutical and biotechnology industries' adoption of e-clinical solutions, cost-effectiveness, faster data processing, and improved data quality, and economic development. E-clinical solutions for Phase III clinical trials are projected to become the standard in forecast period.
End Users Analysis
Contract Research Organizations (CROs) Segment Dominates eClinical Solutions Segment. Contract Research Organizations (CROs) is the leading segment in the market for eClinical solutions. CROs play a crucial role in clinical trial administration, and they require a centralized platform for trial data management. E-clinical solutions offer a cost-effective and expedient way to manage clinical trials. Contract Research Organizations (CROs)are essential partners for urology device manufacturers. They offer expertise in regulatory compliance, clinical trial management, and data analysis, helping companies navigate complex approval processes efficiently.
Consumers prefer Contract Research Organizations -provided e-clinical data management solutions. Contract Research Organizations' cost-effectiveness and efficiency have improved customer acceptance. This category has grown due to global consumer adoption of cutting-edge digital health technology. Due to many factors, Contract Research Organizations (CROs) are expected to develop the quickest. This area is rising due to digital technology usage in healthcare, Contract Research Organizations' embrace of e-clinical solutions, cost-effectiveness, efficiency, centralized data administration, and economic development. The trend should continue in forecast period.
Key Market Segments
By Product Type
- Clinical analytics platforms
- Electronic data capture
- Clinical data management systems
- Safety solutions
- Randomization & trial supply management
- Electronic clinical outcome assessment
- Clinical data integration platforms
- Clinical trial management systems
- Electronic trial master file
- Regulatory information management solutions
By Delivery Mode
- Web-hosted
- Licensed enterprise
- Cloud-based solutions
By Clinical Trial
- Phase I
- Phase II
- Phase III
- Phase IV
By End Users
- Pharmaceutical
- Biopharmaceutical companies
- Contract research organizations
- Consulting service companies
- Medical device manufacturers
- Hospitals
- Academic research institutions
Growth Opportunity
Technological advances are transforming the healthcare industry
In today's world, technological advancements have had a profound effect on every aspect of our existence, including the healthcare industry. With the proliferation of electronic devices, the demand for digital medical solutions has steadily increased. eClinical solutions is one sector of the healthcare industry that has benefited significantly from technological advancements. This industry has experienced a significant increase in demand due to the numerous benefits it provides to healthcare providers and patients in forecast period.
Structure of Regulation and Safety Monitoring
In developed economies such as the United States, the growth of eClinical solutions is driven by regulatory framework and safety surveillance. Due to stringent regulatory structures, eClinical solutions have become increasingly popular in developed economies such as the United States. This has increased the demand for eClinical solutions that are compliant with regulatory requirements. Urology device manufacturers are under strict scrutiny to meet patient compliance and regulatory compliance. Staying compliant with evolving regulations is crucial to market entry and product success.
Data Sharing and Tightening Registration Requirements for Clinical Trials
The US Health Department and NIH are strengthening registration regulatory requirements for clinical trials and fostering data sharing. In order to ensure the safety and efficacy of healthcare products, the US Department of Health and the National Institutes of Health (NIH) have implemented measures to tighten clinical trial registration regulatory requirements and promote data sharing. This action has increased demand for eClinical solutions that can assist healthcare providers in complying with these regulations.
Latest Trends
Surge in Drug Development Studies
The eClinical Solutions Market is experiencing rapid expansion due to a number of factors. An increase in the number of drug development studies conducted worldwide is one of the primary drivers. Pharmaceutical companies are significantly investing in research and development, resulting in a surge in demand for eClinical solutions to streamline and accelerate the clinical trial process.
Increasing Clinical Trials Boost
Alongside the increase in drug development studies, there has been an increase in clinical trials. Increased funding, technological advancements, and the development of novel remedies have all contributed to this expansion. Consequently, the demand for eClinical solutions that improve the efficacy forecast period and precision of clinical trials has increased significantly.
Increasing Acceptance in Clinical Research
In clinical research, the use of eClinical solutions has steadily increased. These solutions streamline and automate clinical trial procedures, rendering them more efficient and cost-effective. In addition, eClinical solutions enhance the precision and dependability of clinical data, which is essential for obtaining regulatory approval for new pharmaceuticals and therapies.
Systems for Clinical Data Management (CDMS)
In the future years, clinical data management systems (CDMS) are anticipated to dominate the market for eClinical solutions. CDMS applications are designed to acquire, store, and manage clinical data from multiple sources. They facilitate the accumulation and analysis of data, allowing researchers to identify trends, monitor patient outcomes, and make informed decisions regarding drug development and clinical trial design.
CDMS Integration with Other eClinical Instruments and Solutions
Integration of CDMS with other eClinical tools and solutions is an emerging trend in the eClinical Solutions Market. During clinical trials, CDMS can be seamlessly incorporated with electronic data capture (EDC) systems, which capture data electronically. Additionally, CDMS can be integrated with electronic patient reported outcomes (ePRO) systems, allowing patients to electronically report symptoms and other health-related data. This integration improves the efficacy and effectiveness of clinical research data collection and analysis.
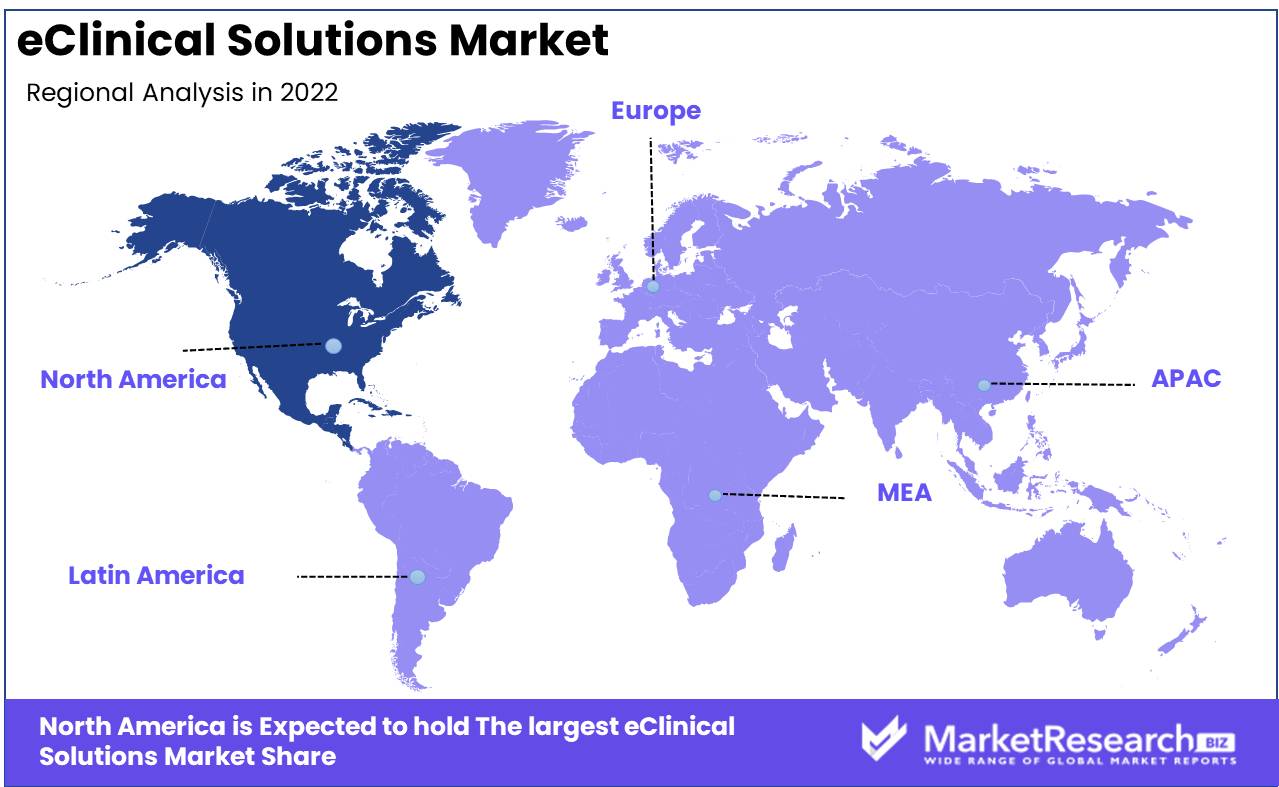
Regional Analysis
The eClinical Solutions Market is led by North America. Extensive research on the global market for eClinical solutions reveals that North America currently dominates the industry. Recent studies indicate that, North America will account for over 51% of the market share, making it the largest revenue source generator on the global eClinical solutions market. This dominance is largely attributable to the increasing number of clinical trials conducted in North America. Several of the world's most influential pharmaceutical companies and clinical research organizations are headquartered in the United States and Canada. These companies are investing significantly in the research and development of eClinical solutions to streamline and improve the efficacy of their clinical trials.
The high level of technology adoption in North America is another important factor driving the region's growth in the eClinical solutions market. It is the ideal environment for deploying eClinical solutions due to its sophisticated infrastructure and high-speed internet capabilities. The adoption of cloud-based technology is also acquiring prominence in this region. This is a result of the increasing demand for remote monitoring and access to real-time data, which has been exacerbated by the COVID-19 pandemic in North America.
Positive government initiatives in North America have contributed to the expansion of eClinical solutions in the region. In the United States, for instance, the 21st Century Cures Act encourages the use of electronic health records and eClinical solutions, thereby fostering market expansion.
In North America, the eClinical solutions market is further segmented into electronic data capture (EDC), clinical trial management system (CTMS), clinical analytics platform (CAP), and randomization and trial supply management (RTSM). Due to the increasing demand for real-time data sharing and analysis, EDC is the most prominent of these segments.
Key Regions and Countries
North America
- US
- Canada
- Mexico
Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
In recent years, the market for eClinical solutions has expanded significantly as the healthcare industry increasingly employs technological solutions to enhance patient care and healthcare outcomes. In this swiftly transforming industry, the main key market players in the eClinical solutions market are driving innovation and establishing a reputation for themselves with their cutting-edge technological offerings.
Oracle Corporation is one of the most prominent participants in this market. Oracle's eClinical solutions enable users to effortlessly manage complex clinical trial workflows and data streams while maintaining data quality and precision. In addition, it provides clinical data management and electronic data capture (EDC) services.
Merge Healthcare's software is used by over 7,000 healthcare government and organizations in over 50 countries. The Merge Health Incorporated company's customers include hospitals, imaging centers, specialty clinics, clinical research institutes, and pharmaceutical firms. In 2015, Merge Healthcare was acquired by IBM. In 2022, IBM spun off its Watson Health business unit, which included Merge Healthcare, into a new company called Merative.
Parexel International Corporation provides a comprehensive range of services for all phases of clinical development, from early-stage clinical trials to late-stage regulatory submissions. Parexel International Corporation also offers a variety of innovative solutions to help its clients streamline the clinical development process and bring new treatments to market faster.
Datatrak International, Inc. is a company that specializes in providing technology solutions for clinical trial management and electronic data capture (EDC). Founded in 1991 and headquartered in Mayfield Heights, Ohio, Datatrak has established itself as a leading player in the clinical research and healthcare technology sectors.
Top Key Players in Eclinical Solutions Market
- Parexel International Corporation
- Oracle Corporation
- Merge Health Incorporated
- Datatrak International, Inc.
- BioClinica
- CRF Health
- ERT
- E-Clinical Solutions
- OmniComm Systems Inc
- Medidata Solution
Recent Development
In July 2022, Oracle Corporation partnered with Microsoft to provide Azure clients with Oracle Cloud Infrastructure databases. This cooperation will provide eClinical Solutions Market organizations a simplified, cloud-based data solution.
IDDI distributes Clario, a leading Randomization and Trial Supply Management (RTSM) Software as a Service provider. Clario can reach new consumers and provide IDDI's clients with unique and effective solutions by expanding their offerings.
In April 2021, Datatrak International, Inc announced Datatrak Direct, an all-in-one iOS and Android data gathering software for ePRO, eCOA, and eConsent. This software allows patients to simply input and submit data to their healthcare professionals, revolutionizing the business.
In February 2021, Parexel a biopharmaceutical research business, teamed with NeoGenomics to improve cancer clinical trial patient selection. This will increase precision medicine in these clinical trials and provide researchers better data to cure cancer.
Report Scope:
Report Features Description Market Value (2022) USD 10.6 Bn Forecast Revenue (2032) USD 32.9 Bn CAGR (2023-2032) 12.3% Base Year for Estimation 2022 Historic Period 2016-2022 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product Type (Clinical analytics platforms, Electronic data capture, Clinical data management systems, Safety solutions, Randomization & trial supply management, Electronic clinical outcome assessment, Clinical data integration platforms, Clinical trial management systems, Electronic trial master file, Regulatory information management solutions)
By Delivery Mode (Web-hosted, Licensed enterprise, Cloud-based solutions)
By clinical trial (Phase I, Phase II, Phase III, Phase IV)
By End Users (Pharmaceutical, Biopharmaceutical companies, Contract research organizations, Consulting service companies, Medical device manufacturers, Hospitals, Academic research institutions)Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Parexel International Corporation, Oracle Corporation, Merge Health Incorporated, Datatrak International, Inc., BioClinica, CRF Health, ERT, E-Clinical Solutions, OmniComm Systems Inc, Medidata Solution Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Parexel International Corporation
- Oracle Corporation
- Merge Health Incorporated
- Datatrak International, Inc.
- BioClinica
- CRF Health
- ERT
- E-Clinical Solutions
- OmniComm Systems Inc
- Medidata Solution




