
Clinical Trial Management System Market By Type (Enterprise, Site), By Delivery Mode (Web-based, Cloud-based, On-premise) By Component (Software, Service), By End-User (Pharmaceutical and Biotechnology Firms, Others), By Region And Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, And Forecast 2023-2032
-
6457
-
May 2023
-
151
-
-
This report was compiled by Correspondence Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Report Overview
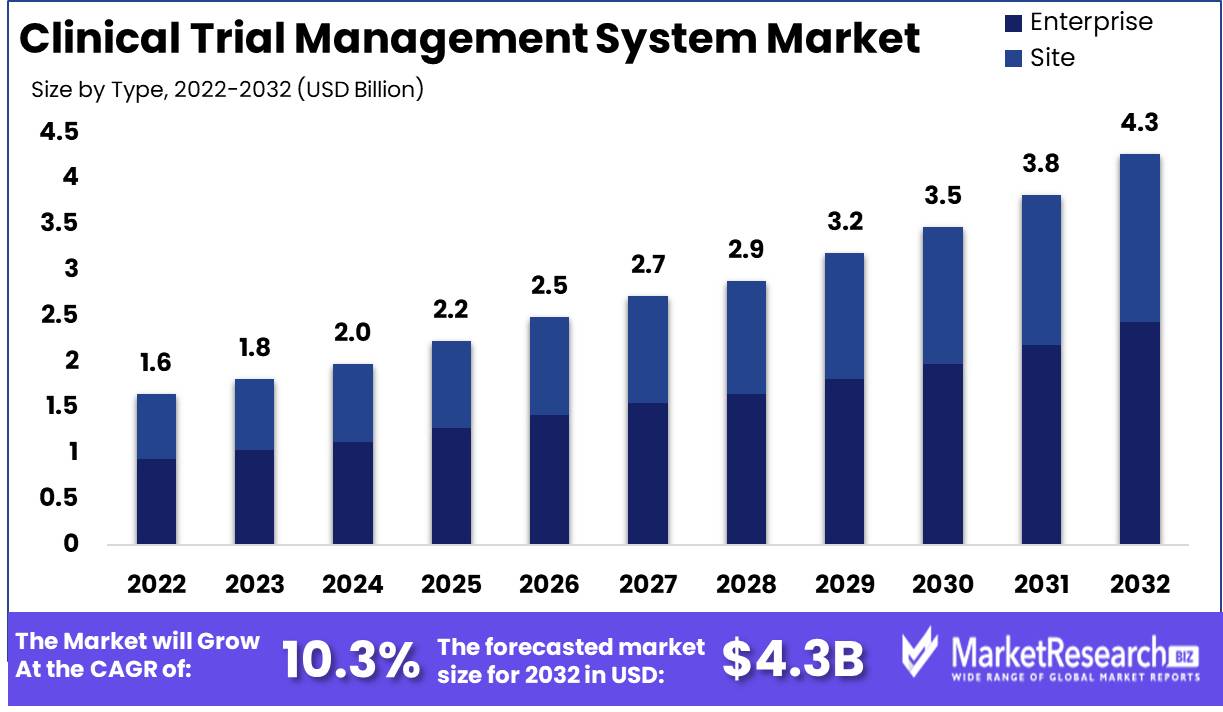
Clinical Trial Management System Market size is expected to be worth around USD 4.37 Bn by 2032 from USD 1.64 Bn in 2022, growing at a CAGR of 10.3% during the forecast period from 2023 to 2032.
The Clinical Trial Management System (CTMS) Market is growing rapidly due to pharmaceutical and healthcare organizations' investments in technological advancements. The management of the increasingly complex clinical trial process must be efficient, effective, and transparent. CTMS provides clinical trial sponsors with a singular platform to oversee the complete process and monitor ongoing trials.
CTMS is software that facilitates and automates the clinical trial procedure. It includes project management, document management, monitoring of clinical supplies, budgeting, and reporting capabilities. CTMS aims to decrease the duration and expense of clinical trials, improve patient recruitment and retention, and ensure regulatory conformance.
Clinical trials require meticulous planning, execution, and monitoring. CTMS assists sponsors in effectively and efficiently administering the process. It enables the accumulation and management of data in real time, ensuring its accuracy and dependability. In addition, it reduces the risk of errors and delays, enhances communication between stakeholders, and ensures ethical and regulatory compliance.
The Clinical Trial Management Systems (CTMS) Market had witnessed significant transformations in recent years, with the COVID-19 pandemic acting as a catalyst for its growth. As pharmaceutical companies and the healthcare sector were grappled with the challenges of conducting clinical trials during a global health crisis, the demand for efficient and streamlined CTMS solutions surged.
This trend is expected to continue during the forecast period as the market witnesses substantial growth. Key players in the CTMS market are vying for an increased market share by offering innovative and integrated solutions that cater to the evolving needs of clinical research. The CTMS Market is poised to flourish, addressing the growing complexity of clinical trials and enhancing their efficiency in the ever-evolving landscape of the pharmaceutical and healthcare sector.
Driving factors
Demanding Drug and Therapy Innovation
The Clinical Trial Management Systems (CTMS) market is experiencing significant growth due to the increasing demand for new drugs and therapies. This growth is closely connected to the rising number and complexity of clinical trials, which has led to a strong focus on reducing trial durations and costs.
The integration of regulatory standards and guidelines into clinical trials has led to the development of efficient and organized clinical trial management systems. These systems leverage advancements in technology and the increasing use of electronic data capture (EDC) to offer valuable insights into patient demographics and disease patterns. They also play a fundamental role in informing important clinical decisions.
Chronic Conditions and Rare Ailments Take Center Stage
Pharmaceutical and biotech companies are increasingly investing in research and development for disease prevention and rare disorders. This has led to the growth of contract research organizations (CROs) and clinical research sites dedicated to advancing knowledge in this field. In parallel, the market is shifting towards cloud-based Clinical Trial Management System (CTMS) solutions, offering real-time benefits while ensuring the security of data.
A Ballet of Innovation and Compliance
CTMS providers need to adapt smoothly to the evolving healthcare regulations, including the 21st Century Cures Act, which has simplified the regulatory approval process for medical devices and pharmaceuticals. This transformation is enhancing patient safety and aligning with ongoing innovations in the healthcare industry.
Restraining Factors
The Multifaceted Obstacles Facing CTMS Adoption
The global expansion of Clinical Trial Management Systems (CTMS) is driven by the increasing complexities of clinical research. However, widespread adoption and effective use of CTMS solutions face several challenges. These obstacles encompass high implementation and maintenance costs, concerns about data security and privacy, a shortage of qualified professionals, strict regulatory demands, and limited awareness and adoption in emerging economies.
High implementation and upkeep expenses
The substantial expenses linked to the implementation and upkeep of CTMS solutions pose a major obstacle. These costs often exceed the budgetary constraints of small and medium-sized businesses. Alongside hardware, software, and technical support expenditures, the costs related to acquisition and configuration significantly contribute to the overall ownership cost. This financial burden limits organizations' ability to invest in CTMS tools.
Security Issues and Privacy Consequences
Concerns regarding the security of clinical trial data present a formidable obstacle. Data confidentiality and integrity must be maintained to prevent reputational harm, legal complications, and monetary losses. The complexity of data security and storage requirements is increased by privacy regulations such as GDPR, HIPAA, and 21 CFR Part 11.
Insufficient Knowledge in Emerging Economies
Emerging economies in Asia-Pacific, Latin America, and the Middle East have limited CTMS awareness and adoption despite their immense potential. In these regions, the lack of understanding of the benefits, the scarcity of trained professionals, and the difficulties of regulatory compliance impede the widespread adoption of CTMS solutions.
By Type Analysis
The Enterprise CTMS Segment dominates the market due to its capacity to manage large operations and provide pharmaceutical and biopharmaceutical companies with customizations. These clinical research organizations are also known for conducting trials in multiple locations, which necessitates a variety of monitoring and management features.
In addition, consumer trends and behaviors tend to favor the Enterprise CTMS Segment. In response to the increasing demand for personalized medicine and precision therapies, pharmaceutical and biotechnology companies must conduct trials tailored to these specific requirements. This necessitates a CTMS with specialized features and a high level of adaptability.
Due to these factors, it is anticipated that the Enterprise CTMS Segment will experience the highest growth rate in the coming years. In this era of personalized medicine and precision therapy, its ability to provide sophisticated features and perform complex operations is crucial.
By Delivery Mode Analysis
Due to its convenience and usability, the Web-based segment dominates the market for clinical trial management systems. This section allows clinical trials to be conducted remotely, without physical presence being required. The market is attracted to its ability to provide real-time data and collaboration features.
Due to its capacity to provide remote access to trials, the economic growth in emergent economies is accelerating the adoption of Web-based CTMS. These nations require a CTMS capable of mitigating the logistical difficulties associated with conducting clinical trials in remote locations. In addition, consumer trends and behaviors are shifting toward the Web-based segment, as participants prefer to participate in trials from the comfort of their own residences.
Due to its flexibility and scalability, the web-based clinical trial management systems market is anticipated to record the highest growth rate over the coming years. As the number of remote trials rises, so does the demand for a CTMS that can facilitate them.
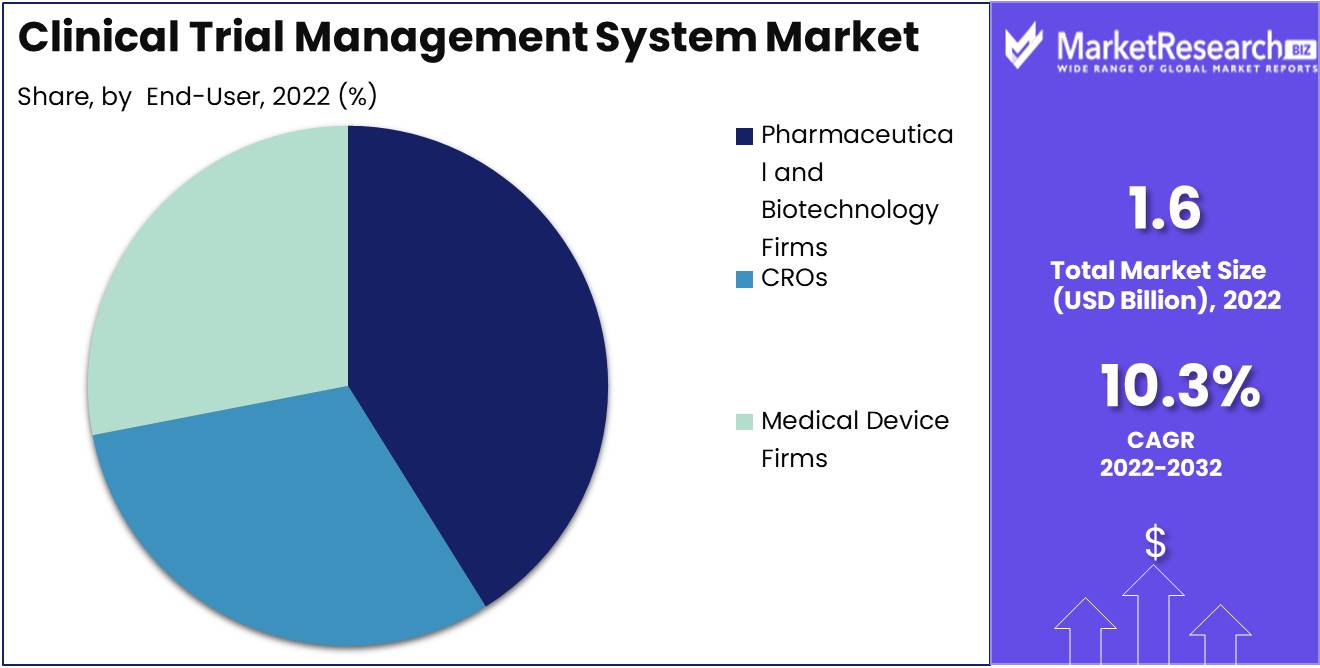
By End User Analysis
Pharmaceutical and Biotechnology Firms Segment dominates the Clinical Trial Management System Market. They need a CTMS that can handle the complexity of these operations, including multiple locations and a variety of monitoring and management features.
Due to the expanding demand for personalized medicine and precision therapies, consumer trends and behaviors are leaning towards the Pharmaceutical and Biotechnology Firms Segment. These businesses are conducting trials tailored to these specific requirements, and thus require a CTMS with customizable features and a high degree of adaptability.
Due to these factors, it is anticipated that the Pharmaceutical and Biotechnology Firms Segment of the Clinical Trial Management System Market will experience the highest growth rate over the next few years. In this era of personalized medicine and precision therapy, the ability to manage complex operations and provide customized features is crucial.
Key Market Segments
By Type
- Enterprise
- Site
By Delivery Mode
- Web-based
- Cloud-based
- On-premise
By Component
- Software
- Service
By End-User
- Pharmaceutical and Biotechnology Firms
- CROs
- Medical Device Firms
Growth Opportunity
Real-Time Data Analytics and Reporting
The escalating demand for real-time data analytics and reporting is a driving force for the expansion of the Clinical Trial Management System (CTMS) market. CTMS's proficiency in real-time data analytics and reporting acts as a catalyst in this context, streamlining the complex clinical development process by providing vital data for monitoring study progress, tracking patient recruitment, and facilitating informed decision-making.
Convergence of Artificial Intelligence and Machine Learning Technologies
The integration of Artificial Intelligence (AI) and Machine Learning (ML) technologies is driving significant growth in the CTMS market. CTMS solutions enhanced with AI and ML automate repetitive tasks within the clinical trial process seamlessly. Leveraging their capabilities allows for the detection of hidden risks, simplification of complex clinical study procedures, and overall improvement in trial efficiency, leading to greater organizational success.
Unveiling the Shift Towards Patient-Centered and Decentralized Clinical Trials
The ongoing shift toward patient-centric trials and decentralized clinical trials (DCTs) represents a transformative approach that prioritizes patient well-being. Patient-centered trials align closely with patient needs and preferences, creating a paradigm shift. DCTs, in contrast, empower patients to take part in trials from their homes or convenient locations, reducing the need for travel to clinical trial sites. CTMS solutions play a crucial role in this era, facilitating real-time data access, smooth communication, and vigilant monitoring, aligning with the principles of patient-centricity and decentralized trials.
The Prodigious Development of Healthcare IT Infrastructure in Emerging Economies
The increasing need for effective clinical trial management systems in emerging economies is a significant aspect of the CTMS market's growth. The development of healthcare IT infrastructure in these regions has led to a strong focus on enhancing the quality of clinical trial data and ensuring compliance with regulations. Additionally, the rising adoption of cloud-based CTMS solutions in these dynamic economies has made clinical trial management more accessible. They have also made it cost-effective, opening up opportunities for a wider range of organizations in the forecast period.
Latest Trends
Combining CTMS and Clinical Research Solutions
A notable market trend arises within the dynamic landscape of clinical trial management systems (CTMS): the integration of CTMS with other clinical research solutions. This trend revolutionizes the process of data collection and exchange, nurturing efficiency, conserving valuable resources, and reducing errors. The integration of CTMS platforms with electronic data capture (EDC) systems is a prime example, as it automates data capture and improves operational efficiency.
Mobile-Based CTMS Applications Are Front and Center
The development of mobile-based CTMS applications represents a paradigm transition in the market for clinical trial management systems. These applications provide clinical trial administrators with mobile access to vital data and reports, thereby enhancing operational efficiency and facilitating well-informed decisions. Mobile-based CTMS applications redefine clinical trial management by facilitating remote work and allowing for location and time management flexibility.
Adoption of Big Data Analytics to Improve Trial Management
The market for clinical trial management systems is illuminated by a brilliant trend: the increasing adoption of big data analytics. A growing number of CTMS platforms include sophisticated analytics capabilities, which provide valuable insights into trial outcomes, patient outcomes, and performance metrics. This integration of analytics enables organizations to make data-driven decisions, uncover concealed patterns and trends, and optimize clinical trial management processes, thereby propelling the industry to unprecedented heights.
Clinical Trial Management at the Dawn of a New Era
The adoption of risk-based monitoring (RBM) is a revolutionary approach to clinical trial management. This innovative method utilizes data analysis and risk assessment instruments to identify high-risk areas within clinical trials, enabling managers to effectively allocate resources. RBM redefines the landscape of clinical trial success by enhancing the efficacy and efficiency of clinical trial management, streamlining the product development process, and fostering cost reduction.
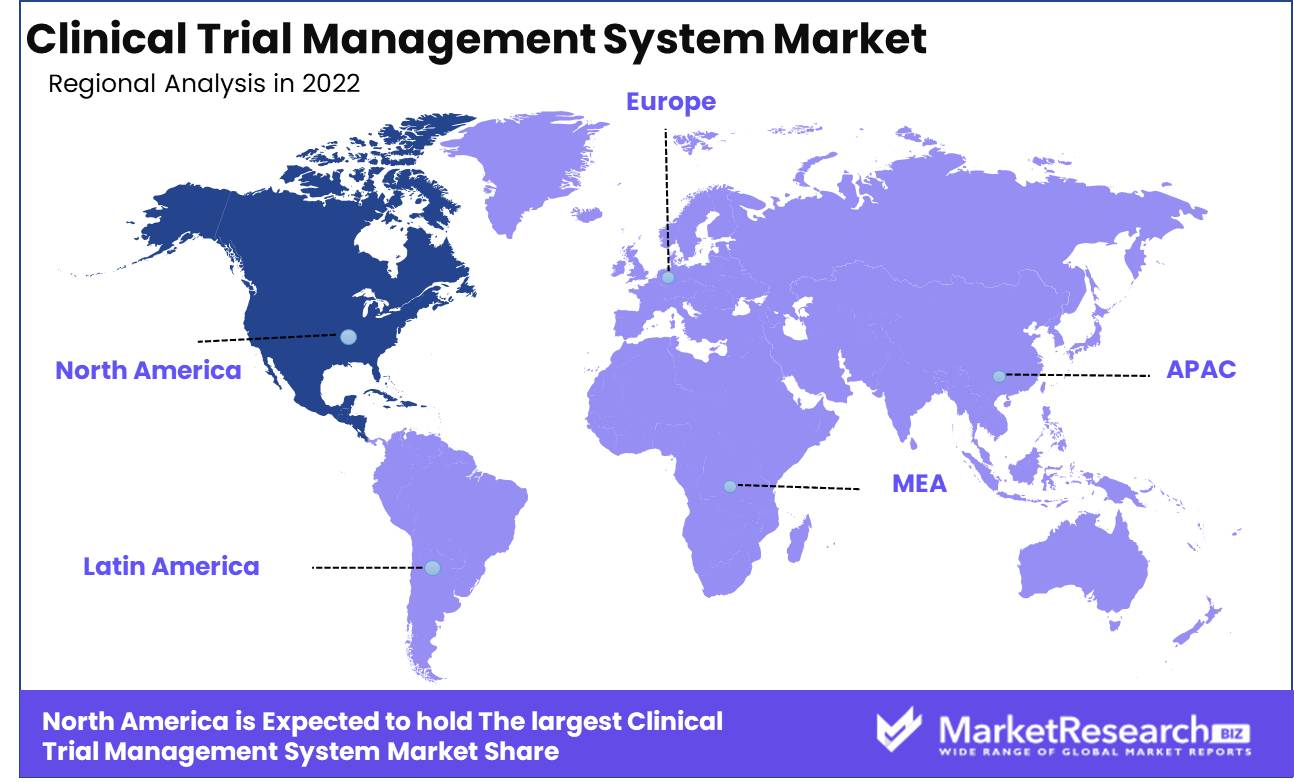
Regional Analysis
In the adoption of CTMS solutions in the biotech and pharma industries, North America, and the United States in particular, has assumed the lead. Clinical trials are costly, time-consuming, and resource-intensive. It is essential for pharmaceutical and biotech companies to streamline clinical trial management in order to ensure the trial's seamless functioning, mitigate errors, and lower operational costs. Here is where CTMS comes into play.
CTMS solutions offer a centralized system for managing clinical trial data, monitoring the progress of studies, managing budgets and resources, and automating workflows. CTMS reduces the need for manual processes and paperwork by providing a single source of truth, enabling teams to collaborate with greater knowledge of study progress, planning, and forecasting, and eliminating the need for manual processes and paperwork.
North America, particularly the United States, has witnessed a significant increase in CTMS adoption due to the rising demand for effective clinical trial management. According to a recent IQVIA report, the United States is responsible for the preponderance of CTMS installations worldwide.
The need for data standardization, data management, real-time monitoring, and cost savings are driving CTMS adoption in the US biotech and pharma industries, according to the report. Faced with intensifying competition, businesses seek to increase efficacy and reduce operational expenses. CTMS solutions offer a viable means of addressing these obstacles.
Key Regions and Countries
North America
- US
- Canada
- Mexico
Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
The exponential growth of the clinical trial management system (CTMS) market is fueled by the increasing demand for efficient and effective management of clinical trials. This industry is comprised of a multitude of key actors who innovate ceaselessly to create advanced CTMS solutions, streamlining every aspect of trial management.
Pharmaceutical companies apply the uttermost diligence to crucial aspects such as study design, data collection, product launches, patient enrollment, and regulatory compliance. Oracle Corporation, Parexel International Corporation, Medidata Solutions, Inc., Bioclinica, Inc., and Merge Healthcare, Inc. are among the market leaders in CTMS. These major players in the pharmaceutical industry invest significantly in research and development in an effort to develop innovative CTMS solutions that improve the overall efficacy and quality of clinical trials.
Oracle Corporation distinguishes itself as a prominent CTMS provider by providing an extensive array of solutions that simplify clinical trial management duties. Their Oracle Health Sciences Clinical One platform combines multiple features, such as electronic data capture, trial management, and clinical data management, into a single platform. This innovative solution unifies disparate functionalities, thereby revolutionizing the clinical trial management landscape.
Additionally, Parexel International Corporation plays a significant role in accelerating drug development and improving clinical trial efficiency. Their ClinPhone RTSM platform allows for real-time supply management, randomization of patients, and drug administration. With its seamless incorporation of essential functionalities, Parexel enables clinical trial stakeholders to more efficiently navigate complex processes and enhance their product portfolio.
Top Key Players in Clinical Trial Management System Market
- Oracle Corporation
- International Business Machines Corporation
- MedNet Solutions, Inc.
- Wipro Limited
- Veeva Systems
- Bio-Optronics Inc.
- Cognizant Technology Solutions Corporations
- Medidata Solutions Inc.
- IQVIA Inc.
- DSG, Inc.
- Forte Research Systems, Inc.
Recent Development
In 2020, Oracle Corporation will release its Clinical One Platform, which provides comprehensive clinical trial management. The platform offers a variety of features, such as data capture, monitoring, and analysis, all in one location, resulting in effective trial management.
In 2021, IQVIA introduced its Orchestrated Clinical Trials solution, which automates and integrates data from various sources to reduce the risk of errors throughout the cycle.
In 2021, Through a collaboration with AiCure, Medidata Solutions, Inc. has also risen to the challenge of offering cutting-edge solutions. They have incorporated patient engagement and data capture technology into their CTMS platform, enabling users to improve data capture and management while simultaneously boosting patient satisfaction.
In 2021, PRA Health Sciences acquired Devana Solutions LLC, a CTMS provider, which they subsequently integrated into their existing systems, thereby streamlining their trial management capabilities.
In 2021, Veeva Systems has recently introduced its Veeva Vault CTMS solution, which is designed to provide end-to-end management of clinical trial operations, thereby entering the CTMS market.
Report Scope
Report Features Description Market Value (2022) USD 4.37 Bn Forecast Revenue (2032) USD 1.64 Bn CAGR (2023-2032) 10.3% Base Year for Estimation 2022 Historic Period 2016-2022 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Type: Enterprise, Site
By Delivery Mode: Web-based, Cloud-based, On-premise
By Component: Software, Service
By End-User: Pharmaceutical and Biotechnology Firms, CROs, Medical Device FirmsRegional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape Oracle Corporation, Medidata Solutions, Inc., PAREXEL International Corporation Company, Veeva Systems, International Business Machines Corporation, MedNet Solutions, Inc., Wipro Limited, Bio-Optronics Inc., Cognizant Technology Solutions Corporations, Medidata Solutions Inc., IQVIA Inc., DSG, Inc., Forte Research Systems, Inc. Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- Oracle Corporation
- International Business Machines Corporation
- MedNet Solutions, Inc.
- Wipro Limited
- Veeva Systems
- Bio-Optronics Inc.
- Cognizant Technology Solutions Corporations
- Medidata Solutions Inc.
- IQVIA Inc.
- DSG, Inc.
- Forte Research Systems, Inc.




