Global Cholera Vaccines Market By Product (Dukoral, Shanchol, Vaxchora, Others Products), By Type (Whole Cell V. Cholerae O1 with Recombinant B-Subunit, Killed Oral O1 and O139), By End-Users (Hospitals and Clinics, Research and Academic Laboratories, Others), By Region And Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends, And Forecast 2023-2032
-
37400
-
June 2023
-
179
-
-
This report was compiled by Correspondence Linkedin | Detailed Market research Methodology Our methodology involves a mix of primary research, including interviews with leading mental health experts, and secondary research from reputable medical journals and databases. View Detailed Methodology Page
-
Report Overview
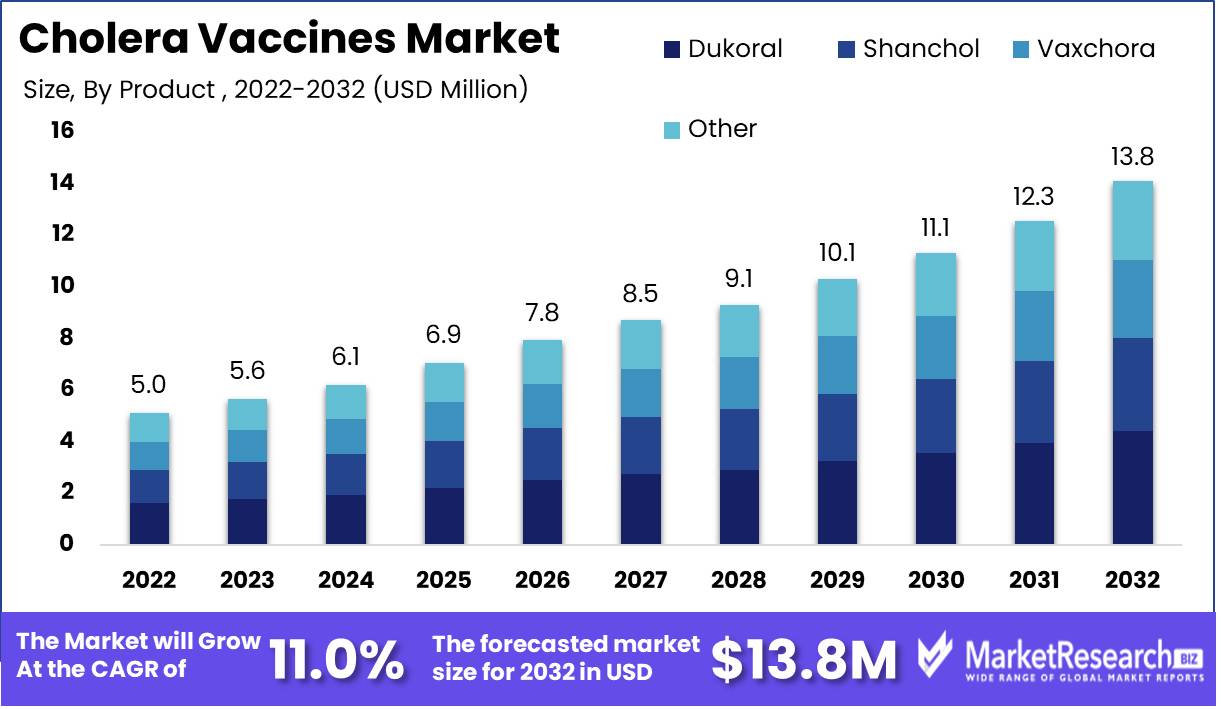
Global Cholera Vaccines Market size is expected to be worth around USD 13.8 Mn by 2032 from USD 5.0 Mn in 2022, growing at a CAGR of 11.0% during the forecast period from 2023 to 2032.
The multifaceted nature of the global cholera vaccines market incorporates a plethora of intricate elements, evidencing its central role in the development, production, and distribution of vaccines to combat and mitigate the dangers of cholera. Cholera, an abhorrent infectious disease caused by the threatening Vibrio cholera bacterium, is primarily transmitted through water and food that have been tainted with toxins. Failure to address this dangerous disease in a timely and effective manner can result in dire consequences, including debilitating dehydration and even premature death.
A primary objective of the cholera vaccines market is to provide safe and effective vaccines to individuals facing the impending threat of cholera, especially in developing countries with limited access to potable water and adequate sanitation facilities.
The cholera vaccine market, imbued with a sacred duty of public welfare, performs an indispensable role in reducing the incidence of cholera and simultaneously saving countless lives. Moreover, prudent investments in the field of cholera vaccine development have the potential to foster remarkable technological advances and innovative discoveries that could have repercussions throughout the entire medical landscape.
Oral cholera vaccines (OCVs) represent a remarkable advancement in this field, exemplifying superior efficacy, easy distribution because they do not require refrigeration and undeniably substantial contributions to the reduction of cholera transmission.
As a result of substantial investments from government agencies, non-profit organizations, and major pharmaceutical corporations, the cholera vaccines market has witnessed a proliferation of commercially available vaccines. Some charitable organizations have deftly woven cholera vaccination into the fabric of their humanitarian efforts, especially in regions beset by natural calamities or virulent outbreaks.
Constant advancements in the fields of research and technology are anticipated to impel the market for cholera vaccines along an inexorable growth curve. In the not-too-distant future, the demand for cholera vaccines may ostensibly decline as the global population obtains access to more hygienic water sources and better sanitation infrastructure.
Unquestionably, the pharmaceutical industry, with its colossal presence, occupies a prominent position in the landscape of the cholera vaccines market, having made substantial investments in vaccine development and production. In addition, non-profit organizations and government agencies exert influence, bolstering the market's momentum with the commendable aim of improving health outcomes.
The ethical and moral dimensions that permeate the cholera vaccine market cannot be emphasized, as they continue to be inextricably woven into its very structure. Among these considerations, the urgent necessity of ensuring equitable access to vaccines, and giving priority to those with the greatest need, is paramount. Simultaneously, the dissemination of accurate and impartial information while avoiding potential conflicts of interest acquires a central position in this ethical discourse.
Driving factors
Cholera Vaccine Market Booms as Demand Skyrockets Due to Increasing Disease Awareness
The Global Cholera Vaccines Market is comprised of ongoing cholera eradication initiatives in various regions. People are now more of the significance of cholera prevention and vaccination due to the rise in disease awareness and education programs. This has increased demand for cholera vaccines and stimulated market expansion.
Advances in Vaccine Technologies Drive Cholera Vaccine Growth
Continual advances in vaccine technologies and formulations are a further factor that drives the market. The efficacy and safety of cholera vaccines have improved significantly as a result of increased research in this field. Governments throughout the world are also promoting the use of vaccines and facilitating their accessibility.
Potential Changes in Regulation Cholera Vaccine Faces Challenges
However, there may be regulatory changes that could affect the market for cholera vaccines. This may be the result of government policies, healthcare system changes, or other external factors. It is essential for manufacturers and suppliers to monitor these changes and take the necessary steps to mitigate risks and maintain business continuity.
Cholera Vaccine Could Be Revolutionized and Emerging Technologies
Emerging technologies such as DNA vaccines, mRNA vaccines, and others may have an effect on the market in the future years. These technologies have demonstrated promising results in clinical trials and have the potential to revolutionize the development and administration of vaccines.
The Cholera Vaccine Market Competitive Landscape Faces Disruption
There may also be potential disruptors in the cholera vaccines market competitive landscape. This may be the result of new entrants, mergers and acquisitions, a shift in market dynamics, or other factors. Companies on the market must be adaptive and flexible in order to remain competitive.
Restraining Factors
Cholera Vaccine Market Growth Is Hampered by Limited Access and Cost
The market for cholera vaccines has been hindered by a number of significant restraining factors. These include limited vaccine accessibility, storage/transportation difficulties, and vaccine reluctance. The limited availability of vaccines is a significant factor affecting the cholera vaccine market. Due to the high cost of production, the insufficient supply of the vaccine, and the lack of awareness in many developing nations, cholera vaccines are not readily accessible to everyone.
Overcoming Vaccine Inaccessibility Key Cholera Vaccine Steps
To address the issue of limited vaccine access, pharmaceutical companies must develop affordable and readily available cholera vaccines. Governments also have a role to play by providing funding to companies that develop vaccines and ensuring that all citizens, including those in remote or rural areas, have access to the vaccine.
Distribution of Cholera Vaccine Overcoming Storage and Transportation Challenges
Cholera vaccines must be stored within a particular temperature range in order to maintain their efficacy. In many developing nations, there is a lack of proper storage facilities and transportation systems that can maintain the required vaccine temperature range. This has caused vaccines to leak, resulting in a significant decrease in the vaccine supply.
Increasing Cholera Vaccine Demand by Addressing Vaccine Reluctance
Investing in the provision of proper storage and transportation facilities is required to address this issue. Pharmaceutical companies that manufacture cholera vaccines should collaborate with government agencies and non-governmental organizations (NGOs) to develop proper storage and transportation facilities in remote and rural areas.
Revenue Decline The Cholera Vaccine Market Faces Obstacles from Uncertain Customers
The adoption of cholera vaccines is being significantly hampered by vaccine hesitancy. Due to skepticism regarding the safety and efficacy of vaccines, individuals avoid receiving them. This has resulted in a decline in demand for vaccines, resulting in a decline in cholera vaccine market revenue.
By Product Analysis
Cholera is a serious waterborne disease caused by the bacterium Vibrio cholerae. If left untreated, the disease can cause severe dehydration and mortality within hours. In recent years, the global market for cholera vaccines has increased steadily. Recent research findings indicate that, among the numerous market segments, the Dukoral Segment is the most dominant.
Dukoral is a vaccine that provides protection against both cholera and an E. coli strain that can induce traveler's diarrhea. This vaccine is a liquid suspension that must be mixed with Sodium bicarbonate solution prior to administration. It consists of heat-inactivated whole V. cholerae O1 bacteria and recombinant B-subunit (rCTB) of the V. cholera toxin. This vaccine is approved for use in adults and children older than two years and is regarded as a safe and effective method of prevention. In the foreseeable future, the Dukoral Segment will continue to dominate the global cholera vaccines market.
The constant expansion of the global cholera vaccines market can be attributed to the economic growth of developing nations. As economies grow, people gain access to improved sanitation and pure water, which reduces the incidence of cholera. However, epidemics may still occur in areas with inadequate water and sanitation. Consequently, the demand for cholera vaccines is likely to rise in these regions, particularly as disease awareness and the availability of prevention tools increase.
Due to its dual efficacy against V. cholerae O1 and E. coli, the Dukoral vaccine controls the global cholera vaccine market. This combination has enhanced the vaccine's value proposition, and more individuals are willing to pay a premium for it. Additionally, the ease of administration through oral dosing facilitates its use, particularly in areas with limited resources and limited access to healthcare. Dukoral's high efficacy and low incidence of adverse reactions also contribute to its popularity.
By Type Analysis
In the market for cholera vaccines, the Whole Cell V. Cholerae O1 with Recombinant B Subunit vaccine is also gaining ground. This vaccine is manufactured using recombinant DNA technology and a heat-inactivated strain of V. cholera O1. The vaccine can be administered either intravenously or orally, making its use convenient.
Similar to Dukoral, it is anticipated that the use of Whole Cell V. Cholerae O1 with Recombinant B-Subunit Segment will increase in regions experiencing economic growth. As countries such as India, Bangladesh, and Nigeria continue to develop, it is anticipated that the incidence of cholera will decrease. However, preventative measures such as vaccination are required to eradicate the disease entirely.
Whole Cell V. Cholerae O1 with Recombinant B-Subunit Segment is acquiring popularity because it provides immunity against V. cholera O1 and, similar to Dukoral, has a low incidence of adverse events. Additionally, more people are likely to take the oral vaccine than the injectable vaccine, which has contributed to the expansion of this market segment.
By End-Users Analysis
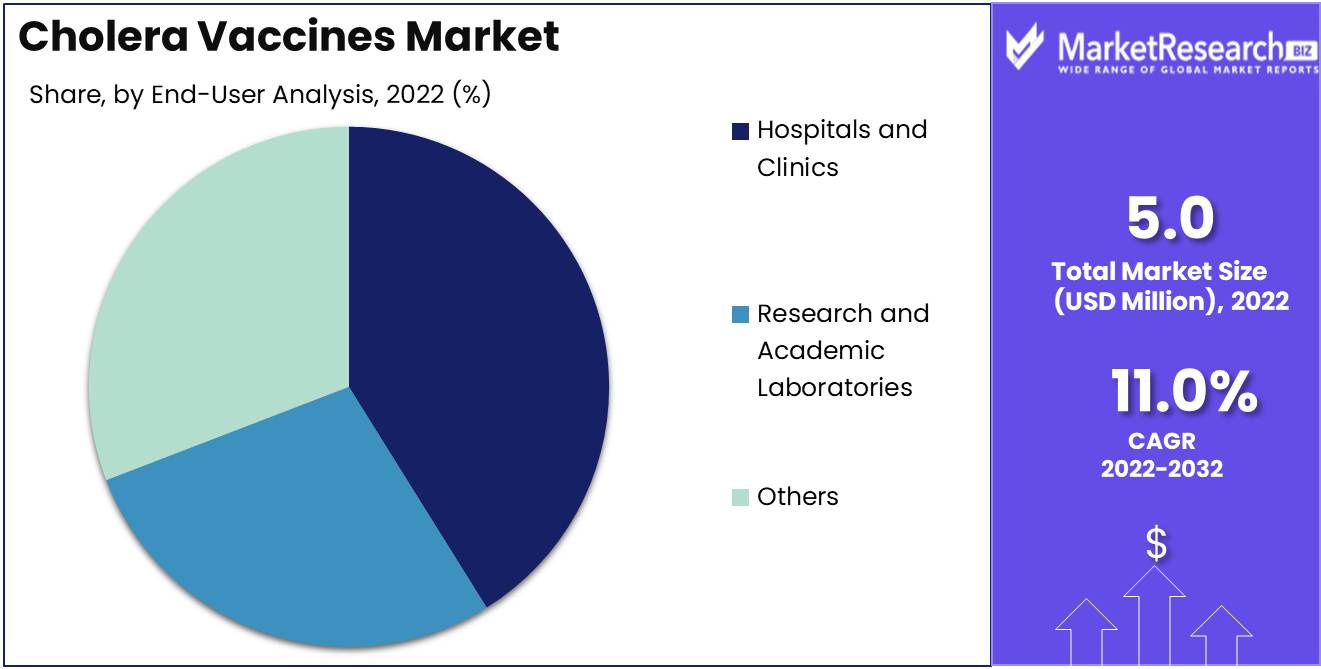
The Hospitals and Clinics Segment dominates the market for cholera vaccines. This is due to the fact that vaccines are primarily administered in hospitals and clinics. Governments and international health organizations also purchase and distribute large quantities of vaccines, with hospitals and clinics being the primary recipients. In these facilities, the demand for cholera vaccines is driven by high accessibility, assurances of quality, and dependability.
As more developing nations invest in their healthcare infrastructure, the demand for cholera vaccines in hospitals and clinics will rise. Governments and non-government organizations have also played a significant role in increasing vaccination rates in these facilities through vaccination campaigns.
The primary driver of the high demand for cholera vaccines in hospitals and clinics is the need for prevention. This awareness of the disease and the significance of vaccination is expected to continue. In addition, rising confidence in the healthcare system has increased the number of individuals who seek vaccination services in hospitals and clinics.
Key Market Segments
By Product
- Dukoral
- Shanchol
- Vaxchora
- Others Products
By Type
- Whole Cell V. Cholerae O1 with Recombinant B-Subunit
- Killed Oral O1 and O139
By End-Users
- Hospitals and Clinics
- Research and Academic Laboratories
- Others
Growth Opportunity
Cholera Vaccines Drive Remarkable Market Growth as Outbreaks Continue
Cholera, an acute diarrheal disease, is responsible for a number of global epidemics, which primarily affect vulnerable populations in impoverished regions lacking access to clean water and sanitation. Oral cholera vaccines (OCVs) are regarded as the most efficient method for preventing cholera outbreaks. In recent years, the market for cholera vaccines has experienced remarkable growth as a result of several initiatives to expand vaccination programs, make vaccines more affordable and heat-stable, and integrate vaccination campaigns with water and sanitation initiatives.
Expanding Global Vaccination Programs to Combat Cholera Outbreaks
The expansion of vaccination programs worldwide is one of the primary growth drivers of the global cholera vaccines market. As part of an integrated cholera control strategy, the World Health Organization (WHO) endorsed the use of oral cholera vaccines (OCVs) in cholera-endemic regions in 2013.
Cholera Vaccine Revolutionized by Affordability and Heat Stability
In the market for cholera vaccines globally, affordability and heat resistance have been significant concerns. Cholera-endemic regions are typically located in low-income countries with limited resources, making costly vaccines difficult to afford. In addition, OCVs typically necessitate refrigeration, which can be problematic in regions where electricity and cold chain infrastructure are unreliable.
Cholera Vaccines Are Now Affordable and Heat-Stable Thanks to Innovative Solutions:
Several initiatives have been implemented to make OCVs more affordable and heat-resistant in response to these concerns. The Gavi Alliance, a public-private partnership that aims to improve access to vaccines for children in low-income countries, has funded OCV campaigns in a number of countries. Additionally, the Serum Institute of India has developed a heat-stable version of the OCV that can withstand high temperatures and does not require refrigeration. This has made remote areas more accessible and transportable.
Integration of Water and Vaccination Efforts Is Crucial for Cholera Control:
The integration of vaccination campaigns with water, sanitation, and hygiene efforts is a further growth driver for the global cholera vaccines market. Cholera is typically transmitted through contaminated water and food; therefore, enhancing water and sanitation infrastructure is essential for preventing outbreaks. Vaccination campaigns can also play an important role in preventing cholera outbreaks, but they must be conducted in tandem with water and sanitation initiatives to have an enduring effect.
Latest Trends
Rapid Growth in the Global Cholera Vaccines Market Caused by Key Trends
The Global Cholera Vaccines Market is expanding quickly as a result of significant market trends like the development of oral cholera vaccines, single-dose formulations, mass vaccination during outbreaks, and research into next-generation vaccines. Cholera is a severe diarrheal disease that is caused by the bacterium Vibrio cholerae.
Innovative Cholera Oral Vaccines Transform Cholera Control Efforts
In the struggle against cholera, the development of Oral Cholera Vaccines has been a significant advancement. OCV is a safe and efficacious vaccine that offers up to two years of cholera protection. Oral administration of the OCV is possible as either a two-dose or a single-dose regimen. During cholera epidemics, the single-dose formulation is strongly recommended. In cholera-endemic regions, the World Health Organization recommends using OCV to prevent and control outbreaks. Consequently, the global demand for OCV is increasing.
On the Cholera Vaccines Market, Single-Dose Formulations Gain Momentum
Single-dose formulations are gaining popularity due to their efficacy and simplicity of administration. Single-dose vaccines are preferred over multiple-dose vaccines because they offer earlier protection and a simpler vaccination schedule. In addition, single-dose vaccines reduce the need for healthcare personnel, which is crucial in areas with limited resources where the majority of cholera cases are reported.
Mass Vaccination During Cholera Outbreaks is Crucial
Another important trend in the global cholera vaccines market is mass vaccination during outbreaks. When a cholera outbreak occurs, it is crucial to vaccinate the afflicted population as quickly as possible to prevent the disease from spreading. Mass vaccination can lessen the severity and duration of a cholera outbreak, prevent new cases, and save lives.
Research on Next-Generation Vaccines Transforms the Fight against Cholera
Research into next-generation vaccines is a significant market driver for cholera vaccines. The next iteration of cholera vaccines is currently in development, and they offer significant advantages over existing vaccine options. The vaccines of the next generation are more potent, longer-lasting, and can be administered via alternative routes such as aerosol or nasal spray. Given the increasing industry antibiotics resistance of the Vibrio cholera bacterium, the development of next-generation vaccines is crucial.
Regional Analysis
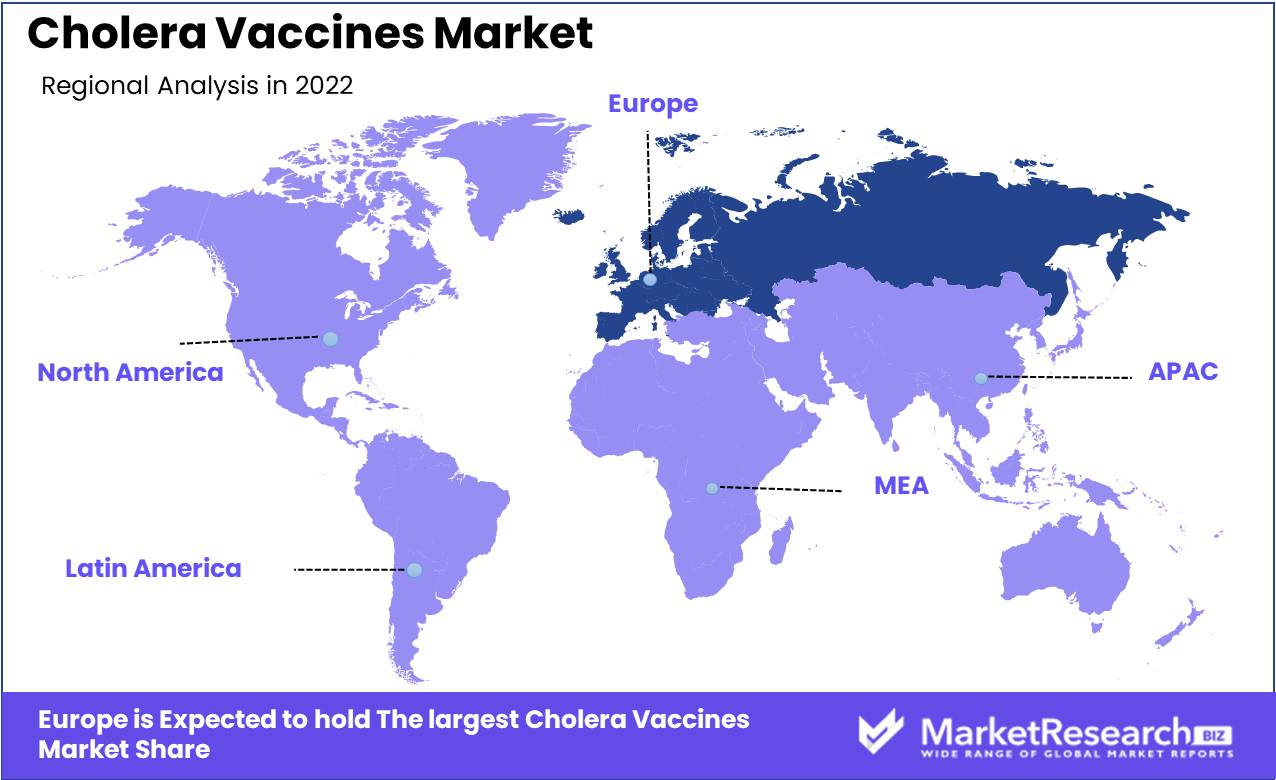
Europe Dominates the Cholera Vaccines Market. In recent years, concerns about the spread of cholera, a bacterial infection that affects the small intestine, have increased. An estimated 4 million cases of cholera occur annually on a global scale, resulting in between 21,000 and 143,000 deaths.
Cholera is predominantly transmitted through contaminated food and water. The World Health Organization (WHO) estimates that up to 80% of cholera cases can be prevented through measures such as access to clean water and sanitation, but vaccination continues to be an essential weapon in the fight against the disease.
The killed whole-cell vaccine was created in the 1940s and is therefore the elder of the two. It is produced by killing cholera bacteria and then using the deceased bacteria to stimulate the immune system. The vaccine is administered in two doses, separated by a few weeks, and provides protection for up to two years. In regions where cholera is endemic, the slain whole-cell vaccine is utilized more frequently than in regions where cholera outbreaks are intermittent.
The oral cholera vaccine, on the other hand, was developed in the 1990s and is a more recent vaccine. It is composed of attenuated, live cholera bacteria and is administered orally in one or two dosages. The vaccine provides protection for up to five years and is typically administered in response to outbreaks, such as cholera outbreaks.
Europe dominates the market for cholera vaccines for numerous reasons. First, Europe is home to a number of the world's leading vaccine manufacturers, such as GlaxoSmithKline and Sanofi Pasteur. These companies have developed and manufactured cholera vaccines for many years and possess a multitude of knowledge and experience in the field.
Second, Europe is home to numerous prestigious research institutions that are actively engaged in the development of new and enhanced cholera vaccines. For instance, the European Vaccine Initiative is a leading organization that works to support the development of new cholera vaccines.
Thirdly, Europe has a robust regulatory framework for vaccines that ensures their safety, efficacy, and high quality. The European Medicines Agency (EMA) is the regulatory body tasked with approving vaccines in the European Union (EU) and ensuring that they satisfy the highest safety and efficacy standards.
Key Regions and Countries
North America
- US
- Canada
- Mexico
Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
The global cholera vaccines market is a rapidly expanding industry that is essential in the battle against cholera, a severe bacterial infection that affects millions of people annually. In order to provide life-saving vaccines to populations at risk of contracting the disease, the key players in this market are crucial.
With its Dukoral vaccine, GSK (GlaxoSmithKline) is one of the leading players in the global cholera vaccines market. This vaccine is efficacious against cholera caused by both the Ogawa and Inaba serotypes and is approved for use in over sixty countries. Sanofi Pasteur is another significant player with its Shanchol vaccine. This vaccine has been approved for use in India and is undergoing testing in additional developing countries.
PaxVax, which manufactures the Vaxchora vaccine, and Bharat Biotech, which produces the Cholera Vaccine Shanchol, are two additional key players in the global cholera vaccines market. There are also a number of lesser players on the market, including EuBiologics, Vietnam Vaccine JSC, and Shanghai Institute of Biological Products.
Top Key Players in Cholera Vaccines Market
- GSK plc (U.K.)
- Valneva SE. (France)
- EMERGENT (U.S.)
- Astellas Pharma Inc.(Japan)
- Sanofi (France)
- EUBIOLOGICS CO., LTD (South Korea)
- Johnson & Johnson Services Inc. (India)
- Celldex Therapeutics. (U.S.)
- Merck & Co., Inc. (U.S.)
- PaxVax
- Other Key Players
Recent Development
- In 2022, The Food and Drug Administration (FDA) granted CVD 103-HgR a license to be used in the United States.
- In 2021, A study published in Nature Microbiology demonstrated that the CVD 103-HgR vaccine was 85 percent effective at preventing cholera in adults.
- In 2020, The WHO advised cholera-endemic countries to incorporate Shanchol into their national immunization programs.
- In 2019, A study published in The Lancet demonstrated that a single dose of Shanchol is 65 percent effective in preventing cholera for up to five years.
- In 2018, Shanchol, an oral cholera vaccine developed by the Serum Institute of India, was prequalified by The World Health Organization (WHO).
Report Scope:
Report Features Description Market Value (2022) USD 5.0 Mn Forecast Revenue (2032) USD 13.8 Mn CAGR (2023-2032) 11.00% Base Year for Estimation 2022 Historic Period 2016-2022 Forecast Period 2023-2032 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product (Dukoral, Shanchol, Vaxchora, Others Products), By Type (Whole Cell V. Cholerae O1 with Recombinant B-Subunit, Killed Oral O1 and O139), By End-Users (Hospitals and Clinics, Research and Academic Laboratories, Others) Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Competitive Landscape GSK plc (U.K.), Valneva SE. (France), EMERGENT (U.S.), Astellas Pharma Inc.(Japan), Sanofi (France), EUBIOLOGICS CO., LTD (South Korea), Johnson & Johnson Services Inc. (India), Celldex Therapeutics. (U.S.), Merck & Co., Inc. (U.S.), Other Key Players, Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) -
-
- GSK plc (U.K.)
- Valneva SE. (France)
- EMERGENT (U.S.)
- Astellas Pharma Inc.(Japan)
- Sanofi (France)
- EUBIOLOGICS CO., LTD (South Korea)
- Johnson & Johnson Services Inc. (India)
- Celldex Therapeutics. (U.S.)
- Merck & Co., Inc. (U.S.)
- Other Key Players




